Introduction: what does moisture-wicking mean?
Moisture management is the process whereby fabrics enable perspiration (sweat) to move from our skin through our clothing layers and finally, into the environment.

It is a fraught journey with iterative rounds of condensation, evaporation, and even freezing throughout. Moisture-wicking is part of how this journey is accomplished.
Many of those who spend a lot of time working or recreating outdoors have made an effort to learn how to dress to try to achieve effective moisture management.
We typically accept that moisture-wicking clothing (including base layers and underwear) is the best way to accomplish moving sweat (moisture) away from the skin and into outer layers where it can evaporate. This process helps keep us comfortable and dry during cold weather.
The best moisture-wicking performance apparel is generally made with synthetic fabrics. While natural fibers including merino wool and cotton are used in some layers that are considered to be moisture-wicking, they aren’t optimal. In this article, we will focus on polyester base layers. In a future article, we will evaluate the performance of merino wool in comparison with polyester.
We know that moisture management does not always work very as we expect during high levels of exertion and cold weather. So we tolerate our moisture-wicking garments becoming wet (or even saturated). And we accept that dry skin and dry clothing layers may have to wait – until the next major innovation in moisture-wicking clothing is announced.
This article deals with the subject of wicking in base layer fabrics and specifically asks the question: how does moisture-wicking work?
When this article was initially conceived, the plan was to conduct wicking tests of many fabrics and garments to see how they compare. But, as I started looking at Polartec fabrics, I discovered discrepancies between marketing claims and the results of my moisture-wicking tests. So, I turned my attention to trying to investigate the accuracy of the marketing claims by adding two additional types of wicking tests and evaluating a larger variety of Polartec fabrics. After we discuss the fundamentals of wicking, this article focuses on the evaluation of Polartec fabrics and what was found.
The manufacturers of moisture-wicking garments claim their products deliver superior wicking performance. That is not necessarily so – wicking claims are like breathability claims. There can be a wide gap in the moisture-wicking performance of different fabrics just like there can be a wide range of breathability performance. Unfortunately, the consumer cannot know what they are buying unless they know how to test for it. In this article, I will describe how end users can conduct simple but useful wicking tests using a very low-cost device.
In this article, I will not dwell on the effectiveness of moisture-wicking as a tool to meet the goal of dry skin, dry clothes, and comfort. Rather, I will be discussing how moisture-wicking works and the abilities of specific Polartec fabrics (and a few non-Polartec fabrics) to move sweat away from the skin on its journey to the environment outside of our clothing. The effectiveness of wicking and alternative moisture management techniques to promote dry clothes and skin will be discussed in a future article.
How does moisture-wicking work?
Moisture-wicking is a process of immense importance in our lives. Without wicking, most plant life would not exist. From small shrubs to giant redwood trees, wicking transports water and nutrients from the ground to every portion of the plant structure including the tallest limbs and farthest leaves. Wicking makes candles burn. A candlewick, on its own, will burn and turn into ash in seconds. Paraffin wax, the main ingredient of candles, is made from hydrocarbons and is difficult to combust. However, by placing an absorbent fiber wick of the right diameter into a block of paraffin wax, you can sustain a flame for hours without burning the wick. In a candle, the wick is covered with wax. Upon lighting the wick, the wax melts and then vaporizes. The flame consumes vaporized wax, not the wick. The heat from the flame causes adjacent wax to melt and flow up the wick. As the melted, flowing wax nears the flame, it vaporizes to maintain the flame. All of this is due to wicking. Of course, wicking can also be a critical component in moving sweat away from our skin to a place it can evaporate, leaving our skin, in theory, dry.
To appreciate the wicking process, you need to understand the science of what supports wicking. In order to avoid putting readers to sleep with a technical explanation of this, I will turn to professionals in the following videos who provide easy-to-understand explanations of the processes involved and even provide a wick rap for those best served by earworms.
- Wicking Fiber Video – this video illustrates how moisture-wicking and capillary action works
- Moisture Wicking Clothing Explained – this video presents the relationship between capillary action and evaporation and how they work together to drive the wicking process in concert with both hydrophobic and hydrophilic fibers
Before going further, make sure you understand the concept of cohesion, which is the molecular electrical force that bonds water molecules into a drop, and adhesion, which is the molecular electrical force that bonds water molecules to non-water molecules (including fabric material fibers). If you’d like, watch the videos twice and absorb all the wicking goodness.
We have learned from the first video that water molecules have a slightly negative charge. They will be attracted to and cling to non-water molecules (fiber surfaces) that have a positive charge. The stronger the positive charge of the non-water molecule, the stronger will be the attraction of water molecules to non-water molecules.
We can see from the videos that wicking requires a capillary. A capillary can be an actual tube that is solid all the way around. A capillary can also be a construction that performs electrically like a tube, i.e., water molecules surrounded by molecules that are positively charged. In moisture-wicking fabrics, capillary tubes are formed by multiple parallel fibers within a yarn. In a moisture-wicking fiber, the yarns have multiple fibers whose spacing is maintained by twisting the fibers together. The tighter the twist, the smaller the spaces between the fibers. Tighter twists can support higher wicking pressure. It will move water farther along the capillaries. Looser twists can move larger volumes of water but for lesser distances. Figure 1 illustrates wicking fibers.

In Figure 1 we see yarns that are knitted together. The yarns consist of bundles of individual fibers. In this example, the bundles are somewhat loosely formed and contain minimal twisting. These yarns are designed to move large moisture volumes a short distance. This type of yarn is present in all of the face sides of the Polartec moisture-wicking fabrics examined in this article. The face side of the fabric is oriented away from the skin; the fabric side that is oriented toward the skin is called the back side or skin side).
Hydrophobicity vs. Hydrophilicity
Two more words mentioned in the videos must also be understood: hydrophobic and hydrophilic.
Hydrophobic (water-fearing) fabrics are water repellent. If you squirt a drop of water at a hydrophobic fabric, water beads on the surface and stays there, just like you see in the photograph at the beginning of this article. Hydrophobic fibers have a strong negative charge – they will repel negatively charged water molecules. Raw (untreated) synthetic fibers tend to be hydrophobic.
Hydrophilic (water-loving) fabrics are water absorbent. If you squirt a drop of water at a hydrophilic fabric, it will be absorbed into the fabric. Hydrophilic fibers will have a positive molecular charge and will attract water molecules. Cotton, wool, and other natural fibers tend to be hydrophilic.
Contact Angle
The degree of hydrophobicity and hydrophilicity will vary amongst fabrics. There is a continuum in water attraction to, or repulsion from, a solid material that is described by a concept called contact angle. Contact angle permits visualization of those forces by simply observing a drop of water applied to the surface.
When a drop of water is placed on a surface, the amount of adhesive force from the surface material determines the shape of the drop. If the surface is highly hydrophobic, the surface adhesive force is far weaker than the cohesive force of the water molecules forming the drop. In this case, the drop will be spherical or nearly spherical (the force of gravity can provide some flattening at the bottom of the drop).
If the surface is highly hydrophilic, the surface adhesive force is far stronger than the cohesive force of the water molecules forming the drop. In this case, the drop will flatten and spread out as the water molecules travel over one another to reach the highly adhesive surface molecules.
The difference in drop shape due to the strength of adhesive forces is described by the contact angle.
The figure below provides a Contact Angle diagram. In each case, the drop is red. The green line shows the contact angle that corresponds to the drop deformation due to the level of adhesion. Adhesion increases from Drop A to Drop C, so A is most hydrophobic, and C is most hydrophilic.
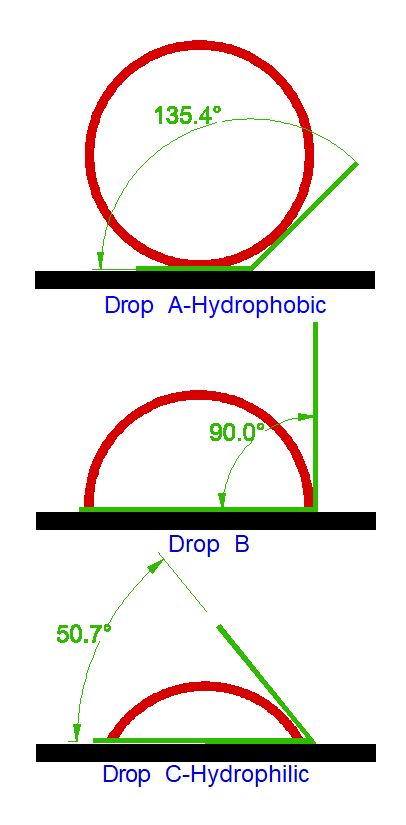
In general, a contact angle below 90° is considered hydrophilic and a contact angle above 90° is considered hydrophobic.
Let’s consider real-life examples in the photograph below:

The red fabric is a Neoshell fabric. It is treated with DWR to render its surface highly hydrophobic. As a result, the left drop of water is nearly spherical. The contact angle, viewed at the bottom of the drop, is well over 90°. The flattening at the bottom is due either to the force of gravity or a low level of adhesive force. One way to determine this is to invert the surface. If the drop remains attached, there is some adhesive force. If the drop slides off, the flattening is likely due to gravity.
The right drop is visibly flat. The contact angle is very low, perhaps 20°. We can see that this surface is hydrophilic. The water molecules show a high level of adhesion to the metal surface. In fact, I flipped the metal strip upside down and the drop remained in place! If you think metal is necessarily hydrophobic, think again.
It is not absorbent, but it need not be hydrophobic.
By observing contact angle from a drop applied to a garment, you can immediately have a good understanding of whether the surface is hydrophobic or hydrophilic. Below, we will discuss the concept of wetting. The flatter the drop, the faster wetting will occur and the more readily wicking will proceed. The rounder the drop, the slower wetting will occur and the greater the difficulty in supporting wicking.
Don’t expect contact angle to remain the same over the life of a garment. It is affected by contaminants, wash cycles, changes in surface roughness, and more.
The photograph at the beginning of this article is a Patagonia base layer made from Polartec Power Dry. It has been washed many times over the years. It is now hydrophobic. I doubt it started that way, but back then, I was not checking my garments’ hydrophobicity and hydrophilicity with a dropper.
Here are some typical contact angles for real surfaces: a paper towel or fabric with excellent wicking properties may have contact angles of 0°. A drop will never form – the water will be instantly absorbed. Untreated polyester might have a contact angle of around 74°. Extremely clean glass can be very hydrophobic with a contact angle approaching 160°
Chemical Fiber Treatments Can Modify Hydrophobicity and Hydrophilicity
Chemical treatments during fiber manufacturing can change the hydrophobicity or hydrophilicity of fibers, allowing the moisture-wicking ability of fabrics to be controlled. A nearly hydrophobic raw material, such as polyester, can be rendered highly hydrophilic with the right chemical treatment. On the other hand, some chemical treatments of cotton can produce a fabric that is very hydrophobic. And of course, we can engineer just about any level of hydrophobicity or hydrophilicity we want.
Moisture Regain and Fiber Moisture Content Capacity
An important characteristic of a hydrophilic material for moisture management is moisture regain. This is the amount of water, by weight of the fabric, that can be absorbed from moisture in the air. Moisture regain is typically very low for synthetic fibers (e.g., polyester is about 0.4% – source). In contrast, the moisture regain for natural fibers (which is dependent on relative humidity) ranges from about 8% to 27% (cotton) depending on relative humidity and about 16% to 30% (merino wool).
The moisture content capacity is the maximum amount of water, by weight of the fabric, that can be absorbed into the fiber. Synthetic fibers have moisture content capacities of 1% to 5%. Natural fibers such as merino wool absorb as much as 60%. of their weight as water into the fiber core.
Why is moisture regain and moisture content capacity important? The higher the moisture regain and moisture content capacity of a fiber, the more moisture will soak into the fibers. Wicking for moisture management is about getting rid of moisture from sweat. The higher the moisture regain or moisture content capacity of a fiber, the more moisture will enter the fiber and the longer it will take for the moisture to evaporate out of the fiber.
Very little moisture can enter a polyester fiber. If the wicking ability of polyester is enhanced through chemical treatment to become very hydrophilic on its surface, it can transport large amounts of moisture with almost no absorption into the fibers. Wool or cotton do not share this property. When wicking large amounts of moisture, a significant portion of the moisture will absorb into the fibers. As a result, drying time for cotton and wool can be substantial in comparison with polyester along with the risk of getting cold when you stop your activity.
It is important to understand the conditions that are necessary to start and maintain water distribution through wicking. Let’s assume that our fabric has sufficient hydrophilicity to support wicking. Before wicking can start, wetting of the fabric must occur. Wetting means that air which is in contact with the fabric surface is replaced with water. If wetting does not occur, wicking will not follow. To sustain wicking, wetting must be continuous. If wetting stops because the moisture supply ends, wicking will end. In order for wicking to occur continuously, evaporation of the water from the garment must also occur at least as fast as water is being moved by the wicking process. Evaporation causes water that is being wicked to leave the fabric. If evaporation does not occur fast enough, fibers will become saturated, and wicking will cease. At that point, you will end up with a wet wicking layer that may take a very long time to dry.
How We Tested
Permeation Kettle Wick & Dry Test
This is a test I developed in 2015. It utilizes my permeation kettles. The test is developed to simulate the interface between skin and fabric. It is a simple test to perform and interpret.
The kettle water is heated to 120 °F (49 °C). A sponge is soaked in 120 °F (49 °C) water and placed on a plastic tray. The weight of the soaked sponge and tray is determined. The sponge and tray are placed on the permeation kettle work surface. The test fabric is weighed and then placed over the top of the kettle. The fabric is pulled sufficiently tight to remove wrinkles. The bottom of the test fabric, which would normally be against the skin, is directly in contact with the wet sponge. The test operates for 1 hour. At the conclusion, the fabric is weighed, and the sponge/tray is weighed. We now calculate the loss of moisture in the sponge to determine how much water was transferred from the sponge into the test item. This is the quantity of water that was wicked. Next, we calculate how much moisture remains in the garment. Finally, we calculate how much water evaporated from the test garment as the test progressed. Evaporation is a critical metric. The more water that is eliminated, the better the fabric performance. Since we have calculated how much water remains in the test fabric at the end of the test, we get an idea of how much water is retained and how much risk might be posed at the end of an activity by using body heat to dry out our garment once our activity is concluded. This test directly addresses a number of issues relevant to our comfort that are not considered in many of the available wicking tests.
The thermal imager is used to produce a time-lapse video that shows the rate and extent of moisture spread due to wicking across the test fabric.
Figures 2 and 3 show the test setup.


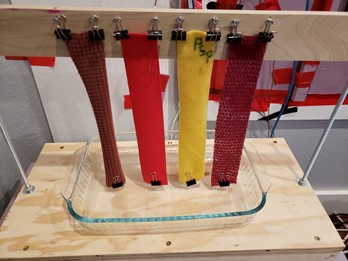
Vertical Wicking Test
The vertical wicking test may be the most popular method of measuring wicking. It is very simple. Hang some fabrics strips into water. Water will travel vertically up the strips. After a predetermined amount of time, the strips are removed, and the travel distance is measured. The strip with the farthest travel is the winner. I have enhanced this test by using the thermal imager to produce a time-lapse video of the process. As wicking occurs, the surface temperature on the wet area drops due to evaporative cooling. The thermal imager allows progress to be easily seen for all fabrics. The wet area can be difficult to see visually on some fabrics. The thermal imager offers another benefit. Fabrics have a face and back. Wicking performance is not necessarily equal on the two. At the end of the test, I can flip the strips and check wicking on the other side. If wicking occurs predominantly on one side, that side will have lower surface temperatures than the poorly wicking side.
There are some problems with the vertical wicking test. The water source is an essentially unlimited reservoir in direct contact with the newly cut bottom fibers. With fibers sitting directly in water, there is no concern for fabric wetting performance – the fabric sample bottom is already sitting underwater so the issue of replacing air around the fibers with water is eliminated. Fabrics that exhibit poor wetting characteristics or uneven wicking on one side or the other will not be identified by the vertical wicking test. Wicking on one side or the other can produce the misleading appearance of excellent performance for the entire fabric. The wicking mechanism here has nothing to do with the skin to fabric transfer that occurs when wearing a garment. As we will see below, the vertical wicking test can cause fleece to appear to be a good wicking fabric. This is only because the vertical wicking test can exploit a wicking mechanism not available in real use. Figure 4 shows the vertical wicking device.
Wetting Test
The first step in establishing wicking is wetting. Wetting is replacing the air surrounding a fabric surface with water. This can happen faster or slower, depending on the fabric contact angle and the fabric construction. In order to better understand the role wetting plays in enhancing or degrading wicking performance, a wetting test can be completed. AATCC TM-79 is a popular example of a wetting test. Most of the popular tests operate in a manner that imparts additional energy (pressure) to a drop contacting a fabric that is not present in the skin/fabric interface during garment use. Such tests can distort the measured performance of fabrics that do not wet easily. In order to avoid what I consider to be a source of mischaracterization for certain types of fabrics, I created my own test that I felt would better represent the skin/fabric interface.
This is another very simple test. Three 4 x 4 inch (10 x 10 cm) samples of a test fabric are produced. Each is weighed. A saturated paper towel is placed on the bottom of a dish. Each individual sample is carefully placed on the surface of the saturated towel to avoid placing pressure between the sample and the paper towel. The sample sits for 30 seconds. At the end of 30 seconds, the sample is removed and weighed. The weight gain for each sample is calculated. The average dry weight and wet weight for the three samples are calculated. A sample with excellent wetting will show a weight gain that is significantly higher than the dry weight. A sample with poor wetting may show no weight gain at all.
A fabric that does well in the wetting test may still not provide exceptional wicking performance. A fabric can be very fast to wet but then lack the capillary capacity to move a great deal of water. This can be seen in some of the wicking specific fabrics included in this study such as the Polartec Silkweight Power Dry sample.
Figure 5 shows the wetting test setup.

Dropper Test
Placing drops on a fabric can provide a preliminary understanding of the speed of wetting and the possible extent of wicking performance. Even more information can be gleaned by adjusting the size of the drops. This is best done with an adjustable pipette. A hydrophobic or poorly wetting fabric will result in droplets remaining on the surface of the fabric. A hydrophilic fabric will absorb the drops. The better the wetting, or the more hydrophilic the fabric, the faster the drops will be absorbed into the fabric.
Standard AATCC TM-79 describes how drops can be applied for this test. Anyone wishing to have a low-cost, quick assessment of wetting and wicking performance can acquire an adjustable pipette online. I recommend the 10-100 µL version. When you get it, practice for a few minutes, watch the training video that is on the supplier’s website and have at it. The pipette is shown in Figure 6.

Now, let’s look at some of these tools in action.
Test Results: Video Observation of Wicking Performance
Dropper Test
In this test, we see an example of fabric with poor wetting performance (Polartec Power Grid) versus another fabric with excellent wetting performance (Polartec Power Dry). The poor wetting performance of the first fabric results in unreliable wicking performance.
Member Exclusive
A Premium or Unlimited Membership* is required to view the rest of this article.
* A Basic Membership is required to view Member Q&A events




Home › Forums › Do moisture-wicking fabrics work?