Introduction
The big question with heat-exchanger (HX) pots is whether the much-vaunted improvements in gas efficiency are worth the extra weight of the pot. That is, given some weight saving in gas, how many litres of water must you boil to compensate for the heavier pot? This article attempts to answer that question using a test apparatus that I built myself to ensure accurate, real-time data collection.
Background
Some time ago Backpacking Light Member Dan Y bought some very inexpensive HX hard-anodised aluminium (HAA) pots from eBay, and sent a couple of them out for review. (I was one recipient: thank you Dan.)
The details for those interested:
- Volume: 1.2 L
- Weight: 174 g without handles or lid (270 g with steel handles & plastic lid)
- MSRP: USD $17.30 with free shipping from eBay at the time of writing.

I love measuring things, so I decided to resurrect the electronic measurement system I had used for my series on Carbon Monoxide Testing of Stoves. This allowed me to measure several data streams at once under laboratory conditions: water temperature, canister weight, and some other temperatures. I could then test several different pots under different conditions. For this, I used a new experimental remote invertible canister stove (V4) which I was working on at the time. More on that another day.
Methodology
Testing Apparatus

I could of course just used a stopwatch for the time and some scales to weigh the gas canister before and after the event, but I prefer to see what is going on during the process. The sort of results obtained are shown further on.
The Pots
- HAA HX pot sent by Dan: 1.25 L, 130 mm dia x 100 mm height, 174 g
- Same HX pot but with fins stripped right off, 144 g
- Small MSR Titan (Ti) pot: 0.85 L, 110 mm x 80 mm, 88 g
- Medium MSR Titan (Ti) pot: 1 L, 140 mm x 65 mm, 82 g
- Large MSR Titan (Ti) pot,: 1.6 L, 160 mm x 185 mm, 116 g
- Large HAA pot: 1.7 L, 150 mm x 100 mm, 123 g
The Titan pots are the ones I use in the field on longer trips. I use an aluminum Trangia kettle for morning tea on day walks.
Test Conditions
There were two different variables here (apart from the pots themselves):
- Flame height or power
- Windshield present or absent
I used 500 mL of cold tap water for each test.
I used Primus Winter gas or Power gas for these tests: apparently the same gas but different coloured canisters. We explored the marketing claims about winter gas already.
The canister was left upright for all the testing. This would have the inevitable effect of varying slowly the ratio of propane to butane from test run to test run, but since the gas consumption was low and the room temperature was >20 C, I did not think this was very significant. The gases do have fairly similar energy content by weight anyhow (mainly from the number of carbon atoms per molecule).
Keeping the flame power the same between test runs is always difficult. I solved this (at least partially) by leaving the stove control valve mostly untouched between test runs and used the safety valve at the other end of the hose to turn the gas on and off. This was only moderately successful as it did not handle testing over a couple of weeks.
In order to avoid jiggling the canister on the load cell, I actually used two hoses for this: one very flexible (yellow) hose went from the canister to a fixed (black) dummy connector, into which the real stove hose plugged. The very flexible hose isolates the canister from the on/off valve and the stove so there is minimal noise on the data, but I would not use this flexible hose in the field: it would be too prone to damage.
Data Collection
I measured the water temperature in the pot continuously while running a stirrer in the pot to get the best measurement of water temperature. I kept a lid on the pot during each test run. I measured the weight of the canister on a load cell continuously as well: by subtracting the starting weight of the canister from each measurement I could tell how much gas had been used. I also monitored the temperature of the canister and of the stove body during each run.
I calibrated the load cell before each test run by adding a laboratory-grade 50 g mass to the canister and looking at the difference.
I calibrated the temperature sensor using cold water measured with a laboratory-grade glass thermometer for the low end and boiling water for the high end. You can’t use ‘hot’ water for this as the steam coming off the water means it is steadily cooling down, but ‘cold’ water is reasonably stable.
It was interesting to note that it was possible to record a temperature a small fraction of a degree above the boiling point (100 C) when the pot was boiling vigorously. This was a real effect, due to the bubbles of steam which sometimes enveloped the temperature probe: that steam was definitely just above 100 C. Cutting off the gas dropped the recorded temperature back to 100 C quite smartly before it started a slow decay, confirming this diagnosis.
The data logging was done with a LabJack T7 Pro logger with the LabJack software (thank you Toby and Christie at LabJack). The device and software run beautifully. It was hooked up to a PC running Windows 7 and the data was analysed using Microsoft Excel 2007.
Each pot was covered by the same flat steel lid (visible above in the photo of the test apparatus) to prevent loss of steam and consequent cooling. It is well-known that a very low flame with no lid could mean your pot might reach (say) 90 C and never get any hotter. That is one reason why tiny stoves (eg candle stoves) do not work in the field: not enough power. Please note that a ‘real’ lid would more or less seal the top of the pot, but the lid used here has a slot on it for the temperature probe and the shaft of the stirrer. Some heat loss might be expected through this slot, and cooling measurements show this does in fact happen. This is one limitation of the whole experiment.
Results
We will start with some general analysis of the full range of pots before looking closely at the Heat Exchanger pots. Doing so allows us to see more easily where the HX pots fit in.
First of all, here we have the results from a typical test run.
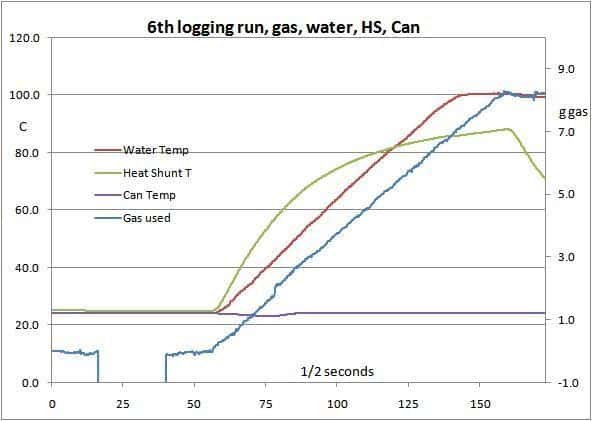
While the results are fairly reliable, there were many variations in test conditions (despite my best efforts), so strict comparisons are not exact. If one did a hundred test runs it might be different – but that would take a lot of time and gas.
The green line shows the temperature of part of the stove body: a matter of interest to me but not relevant here. It starts to fall once the gas is turned off. The flat purple line shows the canister temperature: it stays fairly constant as it is getting some radiation from the stove while it is running and the room is warm.
Flame Height or Burn Rate
Now we look at the range of pots used in this experiment.
The pots used, with their basic parameters are as follows:
Member Exclusive
A Premium or Unlimited Membership* is required to view the rest of this article.
* A Basic Membership is required to view Member Q&A events




Home › Forums › Is a heat exchanger pot worth the weight?