Topic
Modified Kovea Syder for confined pot supports
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Home › Forums › Gear Forums › Make Your Own Gear › Modified Kovea Syder for confined pot supports
- This topic has 63 replies, 8 voices, and was last updated 5 years, 10 months ago by
 DAN-Y.
DAN-Y.
-
AuthorPosts
-
Jan 30, 2019 at 4:33 pm #3575835
Dan, as you well know, system level design and validation is the cornerstone to an efficient cooking system. It doesn’t happen by accident, but months/years of work. That summation of effort is really what amounts to as a trade secret. I highly recommend that you go explore these areas on your own to see what works for best you. Best regards.
Jan 30, 2019 at 4:51 pm #3575836Thank you Jon. I’m in the process of exploring and sharing information. Canister stoves are new to my never ending search for a new efficient cooking system. My Toaks 1300ml pot is working out well being used with the combination titanium windscreen and pot support. My research has led me to the Kovea Spyder stove as an affordable candidate for use in a confined windscreen situation. We’ll see how well it does in my indoor tests. I’ll find out what works best for me and share it here. Thanks again Jon.
Jan 30, 2019 at 5:14 pm #3575843I actually found that the Kovea Spider was idea for the things that I was interested in. I like the remote fed/inversion option for cold temperatures. A big plus is the smaller burner head. The combination of that and the fine pitch threads make it ideal for dry baking. Some of the bigger burner heads like the MSR WindPro II were so big that I couldn’t dial the flame output low enough. If you are interested in becoming a Kovea dealer send me a PM and I will send you the contact information. BTW, I would highly recommend that you use the authorized USA versions of the Kovea Spider as the gray market versions (eBay) have had reliability issues. My 2 cents.
Jan 31, 2019 at 1:18 am #3575938I actually found that the Kovea Spider was ideal for the things that I was interested in. I like the remote fed/inversion option for cold temperatures. A big plus is the smaller burner head. The combination of that and the fine pitch threads make it ideal for dry baking.
Exactly what I was looking for and so I zeroed in on the Spyder. I did my research and so I’m ready to put the Spyder to the test. Tomorrow if I’m lucky, at least 3 tests.
This quick video shows the Toaks 1300, Titanium Windscreen/pot support and Spyder. I have used the kit this past summer in the wood burning mode and that’s why the nice color :-) I’ll do a video showing how it’s used in the wood mode.

BTW, I would highly recommend that you use the authorized USA versions of the Kovea Spider as the gray market versions (eBay) have had reliability issues.
Thanks for the heads-up on the grey market versions.
Jan 31, 2019 at 2:44 am #3575954Just for future reference for me concerning remote canister stoves.
COMPARISON TABLE
The Ultimate Multi-Fuel Stove Comparison Table
W = white fuel, U = unleaded petrol, K = kerosene, D = disesel, C = canister (butane/propane gas)
# Brand Stove Weight Ounces Boil time W U K D
C Simmer Nozzles Features Cost Price check
1 Primus Multifuel III 436g 15oz 3.6 mins Y Y Y Y Y Y 3 Burns all fuel types £143
Amazon – UK shops2 Primus OmniFuel II 441g 16oz 3.0 mins Y Y Y Y Y Y 3 Burns all fuel types £171
Amazon – UK shops3 Primus OmniLite Ti 340g 12oz 3.5 mins Y Y Y Y Y Y 3 Burns all fuel types £153
Amazon – UK shops4 MSR Whisperlite 410g 15oz 3.9 mins Y N N N N N 1 ShakerJet cleaner £119
Amazon – REI – UK shops5 MSR WhisperLite International 441g 16oz 3.5 mins Y Y Y N N N 2 ShakerJet cleaner £105
Amazon – REI – UK shops6 MSR WhisperLite Universal 549g 19oz 3.5 mins Y Y Y N Y N 3 ShakerJet cleaner £153
Amazon – REI – UK shops7 MSR DragonFly 510g 18oz 3.5 mins Y Y Y Y N Y 2 ShakerJet cleaner £135
Amazon – REI – UK shops8 MSR XGK-EX Expedition Stove 489g 17oz 3.5 mins Y Y Y Y N N 2 ShakerJet cleaner £155
Amazon – REI – UK shops9 Optimus Nova 460g 16oz 3.5 mins Y Y Y Y N Y 1 Magnet cleaner, one nozzle £127
Amazon – REI – UK shops10 Optimus Nova+ (Plus) 430g 15oz 3.5 mins Y Y Y Y N Y 1 Magnet cleaner, one nozzle £127
Amazon – REI – UK shops11 Optimus Polaris Optifuel 475g 17oz 3.4 mins Y Y Y Y Y Y 1 All fuels through one nozzle £138
Amazon – REI –12 Kovea Booster Dual Max 434g 15oz 3.5 mins Y N N N N N 1 £157
Amazon – REI – UK shops13 Kovea Booster+1 530g 19oz 3.3 mins Y N Y N Y N 1 £96
Amazon – REI – UK shops14 Kovea Hydra 422g 15oz 3.5 mins Y N N N Y N 1 Low noise Amazon – REI – UK shops
15 Edelrid Hexon Multifuel 330g 12oz 3.1 mins Y Y Y Y Y N 1 All fuels through one nozzle £139
Amazon – UK shops16 Soto Muka Stove (OD-1NP) 333g 12oz 3.0 mins Y Y N N N Y 1 No priming required £150
Amazon – UK shops17 ATG Multi-Fuel Stove 480g 17oz 3.0 mins Y Y Y N Y N 1 Shaker jet, South Africa only
18 Pinguin Pyro Y Y N N Y N Czech stove.
19 Coleman Sportster II 878g 31oz 4.0 mins Y Y N N N N 1 All-in-one stove £84
Amazon – UK shops20 Optimus Hiker Plus 1600g 56oz 3.5 mins Y Y Y Y N Y 1 All-in-one stove £199
Amazon – UK shops21 Optimus Svea 123R 550g 19oz 4.0 mins Y Y N N N N 1 All-in-one stove £89
Amazon – UK shops22 Primus Multifuel Kits 236g 8oz 3.5 mins Y Y Y N Y N 3 Converts gas stoves to multifuel £56
Amazon – UK shops23 Trangia Multifuel X2 525g 18oz 3.5 mins Y Y Y Y Y Y 2 Converts Trangia to multifuel £151
Amazon – UK shops24 Go System Gemini Extreme 300g 11oz 3.2 mins Y Y N N Y N 1 Amazon –
25 Go System FlexiFuel 464g 16oz 4.8 mins Y Y N N Y No longer produced
26 Markill Phoenix 426g 15oz 3.3 mins No longer produced
27 Coleman Pulstar 467g 16oz 4.3 mins Y Y N N N Y 1 No longer produced
28 Tomtop Portable Multi Fuel 575g 20oz Y Y N N Y £50
Amazon –
W = white fuel, U = unleaded petrol, K = kerosene, D = disesel, C = canister (butane/propane gas)• Weight: stove and pump combined, does not include fuel bottles (except on all-in-one stoves) or accessories (e.g. windshield).
• Boil time: manufacturer’s reported time taken to boil 1-litre (one quart) of water using white gas.
• Fuels: for an explanation of the different fuel types, see below.
• Simmer: you can control the output to some extent on all stoves but some have a second, separate controls on the base of the unit for fine tuning.
• Nozzles: Some stoves require you to change nozzles to burn different types of fuel. It’s easier if it has just 1 nozzle. (Also called nipples, jets and valves).
–Jan 31, 2019 at 3:35 am #3575969Those prices are a bit unreal, aren’t they? Compared to a BRS-3000T!
And white gas goes whoomp, while kero stinks. Bygone era.Cheers
Feb 1, 2019 at 10:32 pm #3576301Toaks 1300ml pot used.
1 litre/4.226 cups water boiled per testSpeed to boil was the purpose of the tests and also the ability of the Kovea Spyder to perform inside the confined space of the pot support.
Tests were performed in my kitchen under calm conditions.
Stove burner was set on wide open valve. Canister was in upright position.
1st test = 53* start water temp, 10:18 time to boil
2nd test = 53* start water temp, 10:30 time to boil
An observation was made and so I made a modification and resumed tests.
3rd test = 49* start water temp, 9:14 time to boil
4th test = 50* start water temp, 9:14 time to boil
5th test = 50* start water temp, 9: 00 time to boil
6th test = 50* start water temp, 9:21 time to boil
7th test = 49* start water temp, 10:45 time to boil
8th test = 48* start water temp, 12:00 time to boil
Don’t know why the last 2 tests were so different in length of time to boil. Maybe the water temp to start was actually lower. I may have been in a hurry and not given the thermometer ample time to measure correctly. dunno!
Kovea Syder performed well.
Feb 1, 2019 at 11:07 pm #3576304Last 2 tests: Could the canister have been chilling down or getting low in propane? 8 tests in quick succession …
Cheers
Feb 2, 2019 at 1:35 am #3576328So???? Where are the F values?
Feb 2, 2019 at 2:19 am #3576338Not done any timed testing. It’s a notably fast and efficient setup.
Looking at the video i’m wondering how much more-so with a Caldera style pot support/shield. Yours appears to vent excess heat out the side rather than directly on to the pot sides. Whether this makes a big difference i’m not sure.
Feb 2, 2019 at 2:51 am #3576348Where are the F values?
1/60 at f5.6?Cheers
Feb 2, 2019 at 3:23 am #3576349Roger,
So glad that you are supporting the StoveBench metrics that baackpackinglight so graciously recommended.
Best
Feb 2, 2019 at 3:58 am #3576353Notes were taken, adjustments are in process. Tests will resume Monday……after the Super Bowl.

Need more speed. You canister geek guys can figure out the F values with your Stove Bench Protocals when you get to the remote fed canister stove testing.
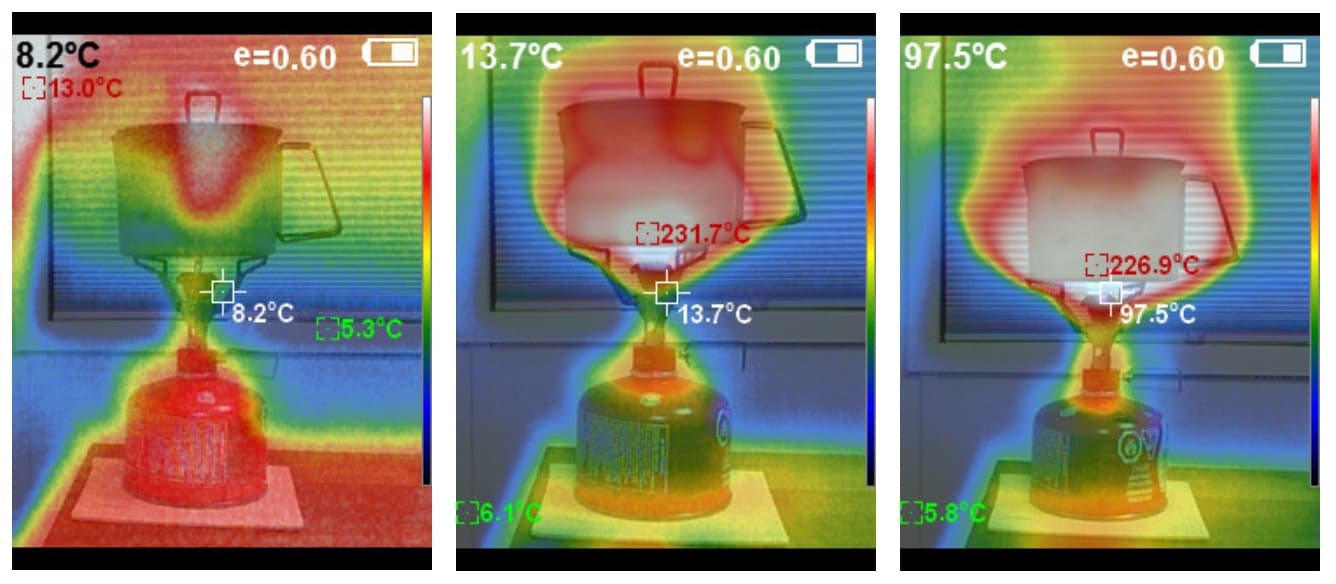
Feb 3, 2019 at 10:09 pm #3576631Some of you may want to know where the heat goes once it leaves the bottom of your pot. Look at Ryan’s infra red photos and see how the heat hugs the sides of the pot as it rises.

I quote Ryan:
On the left, the thermal image shows a stove system with the burner off. In the center image, the same system is operating with the burner turned down about 25% towards its off position, using a nearly-full canister. On the right, the system is in use with the stove throttle turned to its maximum using a nearly-full canister. Note the high amount of wasted heat on the right (full-throttle) image, as indicated by the thermal pattern surrounding the pot. Interestingly, thermal imaging of stoves operated at full throttle on canisters containing less than 80% of their fuel capacity revealed images that looked more like the one in the center rather than the one on the right.
Feb 3, 2019 at 10:18 pm #3576633A canister with <80% capacity has probably burnt off much of the propane already, and is now burning mostly butane. The pressure in the canister will be much lower, so there will be less gas coming out at ‘full throttle’. Further details are at
https://backpackinglight.com/evaporative-heat-loss-upright-canisters-part-4-propane-and-cold-temperatures/ and
https://backpackinglight.com/forums/topic/evaporative-heat-loss-in-upright-canisters-part-4-bombshell/As Rudy G said, ‘full throttle ain’t full throttle’.
Cheers
Feb 4, 2019 at 1:08 am #3576659Roger, in another thread you said:
Now allow the windshield to close in on the pot. This would trap a lot of very hot air around the canister – air at several hundred degrees.
In the infra red photos above, what would your best guess be on the temperature of the rising air on the sides of the pot?
Feb 4, 2019 at 3:21 am #3576680VERY hard to say, as IR cameras are entirely ‘false colour’, and you can adjust the high and low ends of the colour range pretty much to whatever you want.
The flame under the pot will probably be around 800 C to 1000 C. That would be well into the saturation end of the spectrum of course.
The figure of 226.9 C on the pot (RH photo) is equally suspect. Obviously the pot will NOT be at 226.9 C, but the gas in front of it might be. The problem here is the e value at the top, which I take to mean the assumed emissivity used in the colour scale. But you can change that e value (0 to 1) and get very different results, as I found out when preparing Part 5 of my Winter Stove series:
https://backpackinglight.com/caffin-evolution-of-winter-stove-part-5/
It was very hard to get reasonable results when looking at a painted surface AND a TI surface AND a polished aluminium surface in the one frame. Each has a very different emissivity, and an IR camera is not all that smart.If I had to guess, I would punt for ABOUT 250 C in the white region around the pot. But that is a total guess, and I would want a thermocouple reading in the gas stream before committing to anything. I would NOT try to use an LM35 for that!
In short, the photos are good at showing you the gas flow, but cannot be relied on for measurement imho.
Cheers
Feb 5, 2019 at 4:46 am #3576826Thank you Roger. The center photo shows a higher temperature at 25% less throttle that the full throttle photo on the right. Crazy stuff there. It would be nice if I had a thermocuple to test the gas stream along side the pot. In the Stovebench thread someone said there is heat loss from inside the pot going outward when the valve it throttled back to conserve fuel. The pot is absorbing heat as long as the outside temperature going up the side of the pot is above 215 degrees…correct?
Based on what I saw in the center photo and some info I found in the Stovebench thread, I decided to change my test strategy.
In todays tests I used 70 degree starting water temperature and 70 degree ambient air temperature.
I filled 8 one litre bottles with water let stand overnight to acclimate to the 70 degree air temperature in my home. I started testing after the sun set so i could view the flame pattern under the stove. I started the stove at full throttle, saw that flames were shooting out the top air exhaust holes so i throttled back till flames were no longer visible beyond the wall of the pot support. I then marked the valve with a felt tip marker so I could return the valve to that “ideal” valve opening for the 8 tests that were to follow. I then proceeded and these are the results:
Time to boil 1 litre of water: (not interested in amount of fuel used at this time)
- 8:33
- 9:05
- 8:38
- 9:33
- 9:36
- 9:38
- 10:37
- 9:54
I watched water boil :-) again and again…..

.
Feb 5, 2019 at 6:04 am #3576839The very frustrating world of stove testing … we (all) know it well.
An IR camera is very good at showing you the flow pattern for the flames and exhaust gases. Frankly, I think it is pretty useless at measuring actual temperatures. In my Part5 article, I had to adjust the emissivity all over the place to get IR temps to match my measured temps on the very shiny aluminium stove body (LM35s).
The pot is absorbing heat as long as the outside temperature going up the side of the pot is above 215 degrees…correct?
That is 215 Centigrade, not Fahrenheit. The pot would be absorbing energy as long as the gas stream is just a bit above 100 C.Overall, the times you recorded seem to be slowly climbing, and this could be as expected for a cooling canister. I THINK. You could check this by placing the canister in a large bowl of water at 70 C, replenishing the water as it cooled. On the other hand, with 8 runs, I would expect the amount of propane in the canister to be slowly falling, which will slowly change the canister pressure downwards. That means that the flow through the jet will be slowly reducing despite the fixed setting.
:) I found testing remote canister stoves with the canister inverted so much easier. And automatically recording the mass change of the canister at 10 second intervals, along with the water temp, made life also easy. Can I recommend a Labjack T7 data logger? :)
Techie: the LM35 is quite OK for recording temperature (when waterproofed) and you can get little load cells with electronics on the web very cheaply today. Lotsa $$ all the same.
Cheers
Feb 5, 2019 at 2:19 pm #3576872“Crazy stuff there. It would be nice if I had a thermocuple to test the gas stream along side the pot. In the Stovebench thread someone said there is heat loss from inside the pot going outward when the valve it throttled back to conserve fuel. The pot is absorbing heat as long as the outside temperature going up the side of the pot is above 215 degrees…correct?”
Yeah, it looks kind of crazy, but is actually sensible. Even though we tend to look at things as strictly Newtonian in nature, we find that Quantum physics actually plays a big part in stove efficiencies. While we generally take it as an axiom that heat travels from high to low (Newtonian viewpoint) you find that it actually travels the other way (Quantum viewpoint,) too.
Or, It is the OVERALL value that Newtonian physics deals with. Whereas quanta exchange is more accurate and deals with the NET result. You can see this at very low heats. A Liter of 0C water will heat to 50C and never get any hotter. We are quick to explain this by increased vapor pressure, and, adding a top will get another 50%. Then we complain that it still didn’t boil. We didn’t really grasp the whole picture, though. A part of the heat is also being radiated away from the pot. When the two are equal, the system is at equilibrium. Maintaining the same heat input will do nothing except maintain the same temperate of the system, given all else is equal. BUT WE ARE STILL MAINTAINING A HEAT INPUT.Where does the heat go?
The entire surface area of the pot is radiating heat away from the pot, pretty much regardless of the temperature. The hotter it gets, the more IR is radiated. The cooler portions do not radiate as much. Or, we can view this as “the hotter it gets, the more heat we need to add” to get it hotter. At body temperature, we say a pot is neither hot nor cold while holding it. This is not actually correct. We should be saying it is at equilibrium, where gaining heat is equal to loosing heat. (Again, there is a LOT of things that I am ignoring, specific heat, density, phase change, vapor pressure, etc…so this is not entirely correct, for example at low densities an atom can be moving very fast hence is very hot, but it lacks any mass so for a given volume of the space around it can be quite cold in total.)
For a more real example, painting the outside of a pot does not increase heat input…If anything, it acts as an insulator. Why does it seem to work? It decreases heat output (refraction, readsorbtion, reflection of IR.) But, it appears to be the same thing to a Newtonian observer. (Indeed, this is why different colors seem not to effect a painted pots advantage except very slightly.) However to a Quantum observer, he would say “yes,he looses some input heat, but gains MORE heat by not loosing the heat that was already input,” hence a net gain in efficiency. (I would note that darker colors of various makeups tend to absorb some IR, and not really eliminate it from the overall equation, but for this example it works pretty well.)
Many of the effects are minor and play almost no part (like radiated IR from a pot lid or reflected IR from a wind screen.) Some effects are rather significant (like radiation from a pot’s bottom into a canister.) No one bothers with this stuff except at low heats and very high efficiencies.
Instrumentation can easily be fooled by anything more than a single atom thick. So, it is not uncommon to see high pot heat values near the bottom…even in the range of 200-300C. It may indeed be radiating at that temperature regardless of the intervening layers of gasses. Indeed the water temp inside may be well above the boiling point…in fact a quanatum viewpoint accepts this as a given. But again, we look at the entirety or take a Newtonian viewpoint and except a “rolling boil” as correct, though even this is not actually true. Steam can reach much higher temps than 120C, or go to 80C by the time it rises to the surface. Large bubble at the bottom, small or nonexistent at the top. This is NOT a trick of refraction and pressure, though they both play a part. The actual water molecules will oscillate between the two states. And, yep, you guessed it, eventually average out to 100C. But, no single molecule may ever be AT 100C, that is only an average.
Feb 5, 2019 at 10:45 pm #3576978@rcaffin,
The pot would be absorbing energy as long as the gas stream is just a bit above 100 C./212FThat’s what I wanted to hear :-)
Thank you @jamesdmarco and @rcaffin. The two of you are a valuable asset to the scientific minded comunity here. So much of what you say goes right over the top of my head and have to read and re-read what is said in order to understand what you are saying. :-)
My question regarding heat up the sides of the pot was brought about by the statement that @jamesdmarco made in the Stovebench thread. He said:
This pretty much jives with my tests. 7-10 minutes (in that range) seems good for overall fuel efficiency. Longer times means the pot radiates heat fast enough to hurt efficiency.
I’m good with what Roger said about absorbing energy as long as the gas stream is just a bit above 100 C./212F
Now I ask James, regarding your comment in this thread (The entire surface area of the pot is radiating heat away from the pot, pretty much regardless of the temperature. The hotter it gets, the more IR is radiated.)
James, that radiation is from the outside of the pot, the water inside is not radiating heat outward, the water is still absorbing….correct? yes or no :-)Feb 5, 2019 at 11:26 pm #3576997Yes, but … the water could radiate upwards, but that would be mostly blocked IF you have a shiny lid on the pot.
To consider: the amount of IR radiated by an object at 800 C is vastly more than that radiated by the same object at 100 C. Confirmation test: hold your hand in front of said object and ‘feel’ the IR.
Cheers
Feb 6, 2019 at 12:12 am #3577014If I recall correctly, radiant heat transfer is a function of the difference in absolute temperature to the 4th power. 100 C = 372 K, 800 C= 1073 K . The difference in heat transfer is huge.
Feb 6, 2019 at 12:39 am #3577026“Now I ask James, regarding your comment in this thread (The entire surface area of the pot is radiating heat away from the pot, pretty much regardless of the temperature. The hotter it gets, the more IR is radiated.)”
Dan, yes.
“James, that radiation is from the outside of the pot, the water inside is not radiating heat outward, the water is still absorbing….correct? yes or no :-)”
Dan, Not to be a pain in the butt, but Yes and No. Metal absorbs say 99.9999999999999999999999999% of IR radiation (not a correct value.) Some IR manages to slip through directly, though depending on the thickness, density, blah, blah, blah… it is undetectable, generally.
IR is a radiation or enrgy. It absorbed by almost any mass. (Though some is actually transparent to it.) For metals, in general, what happens is it is absorbed, increasing the “heat” state. Heat can be interpreted in various ways: molecular vibratiion, lengthening of electron shells (exitatation), and/or even loss of electrons (ionization) and in extreme heat plasma. Anyway, the heat is retransmitted in a 360 degree probability sphere…some will go back the way it came, some will escape to the next atom. Some of the IR is duplicated as the atom “relaxes” and electrons drop back to their non-excited states. This releases the IR photon to the next atom with the same probability’s all over again. Sometimes it will only release part of the energy as a lower order photon and keeping part as “excitation”. Sometimes it will simply convect to another atom as Brownian movement or molecular “bumping.” Generally we say the pot gets hot. Anyway, there is no set formula for determining which will happen, only probabilities of possible dispositions for the added additional energy. The transfer of heat from water to metal to the outside is NOT instantaneous, though. Conduction is a measure for the SPEED at which this happens. (Metals form a different structure, but still the rules apply.) So looking at the total pot structure, you can envision the heat as being radiated from the outer surface. But it may have been produced from the second or third or fourth or fifth or some other layer of atoms UNDER the surface, not necessarily from the surface layer of atoms. But, assuming the pot has several thousand layers in its thickness, it will be difficult to know the difference. And, the IR will likely be at a different frequency than the IR released from the water, or the IR released from the flame. This is just basic laser physics as far as the frequency and it is based on the composition of the material releasing the photon…skip it…
Anyway, you can see that water and the pot act the same, except, you deal with two different materials. The water still radiates heat. It may be picked up by the other water molecules, and/or the pot. This “starts” the above heat transfers. There is no actual start, though (at least above absolute zero,) just an increase in what is already happening either in the plus (heating) direction or minus (cooling) direction. Newtonian views are usually enough, but they can fail for the last 10-20% of efficiencies. My example with the paint is one such, even though it is only 7-10%.
Anyway, the actual thermodynamics says heat flows in both directions at all times. The water radiates heat and it also absorbs heat. It radiates to the pot as it absorbs from the pot. The pot absorbes from the flame and radiates to the flame. The hotter something is the more it radiates.The difference between the input and output indicates the plus or minus direction of the heat flow (not correct terminology, but easily understood.) Most of the heat produced by a stove starts as IR. It pays to understand it at least in general terms. Is that better, Dan?
Feb 6, 2019 at 12:56 am #3577028Actually, I would guess that 99% of the heat transfer from water to pot is by conduction. Water molecules banging around hit metal atoms in the pot and kick them around. To put it slightly more simplistically.
By the same token, very very excited gas molecules (800 C?) in the stove exhaust hit the pot atoms (80 C?) and kick them around.
And if you pick the pot up when it is boiling, the excited atoms in the metal pot will … excite the cells in your hand, making you ‘excited’.
Cheers
-
AuthorPosts
- You must be logged in to reply to this topic.
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Trail Days Online! 2025 is this week:
Thursday, February 27 through Saturday, March 1 - Registration is Free.
Our Community Posts are Moderated
Backpacking Light community posts are moderated and here to foster helpful and positive discussions about lightweight backpacking. Please be mindful of our values and boundaries and review our Community Guidelines prior to posting.
Get the Newsletter
Gear Research & Discovery Tools
- Browse our curated Gear Shop
- See the latest Gear Deals and Sales
- Our Recommendations
- Search for Gear on Sale with the Gear Finder
- Used Gear Swap
- Member Gear Reviews and BPL Gear Review Articles
- Browse by Gear Type or Brand.