This final part of my canister stove series is more of a technical addendum that addresses the canister stove’s interaction between propane and cold temperatures.
Why isn’t propane very useful in an upright canister stove if you want to use the entire canister at low temperatures?
Given all the ideas in the previous three parts of this series, one has to ask what limitations restrict the use of upright canisters at below-freezing temperatures. It turns out there some significant restrictions. I took three different fuel mixtures and ran them through the spreadsheet used in the article The Effect of Cold on Gas Canisters by Stuart Robb and Roger Caffin to see what would happen to the propane concentration as the gas was used up. The results were a bit surprising.
Description of Data
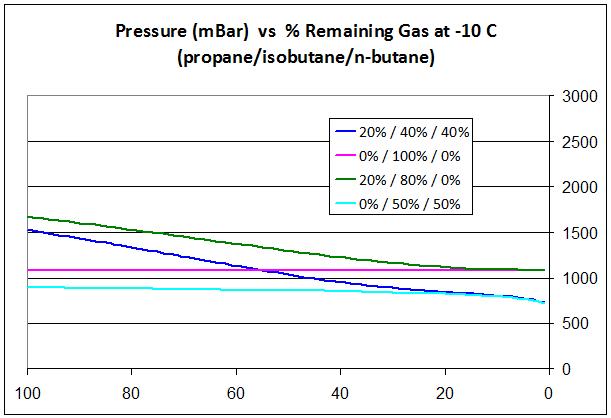
;
The graph here is for canisters at -10 C (14 F). The X axis is how full the canister is: 100 = full canister (at left) and 0 = empty canister (at right). The Y axis on the right is pressure, and 1000 mBar is the nominal pressure at sea level.
The pink line shows how pure iso-butane would behave: the pressure stays constant of course as the composition does not change (it’s all iso-butane).
The green line shows what happens if the canister starts with 20% propane: when it has about 25% left the boost from the propane is all gone. It has boiled off.
The dark blue line shows what you would get with a propane/iso-butane/n-butane blend: the pressure starts higher but by the time the canister has 50% left, the pressure goes below the pure iso-butane case, and below 1000 mBar, so it won’t work (at sea level and -10 C).
The pale blue line shows what would happen with a 50/50 blend of iso-butane and n-butane. It’s always below 1000 mBar so it will never work (at sea level) but this shows how isobutane preferentially depletes in a mixture with n-butane, like propane does, but the effect is much less. There’s a little depletion in the first 90% of the canister (so the boiling point gradually increases a little). There’s a lot of depletion in the last 10% of the canister (so the boiling point will quickly increase to pure n-butane, 31 degree F (-0.5 C)). This is part of the explanation why it’s difficult to burn the last little bit of fuel in a canister.”
Implications
Member Exclusive
A Premium or Unlimited Membership* is required to view the rest of this article.
* A Basic Membership is required to view Member Q&A events



Home › Forums › Evaporative Heat Loss in Upright Canisters (Part 4 – Propane and Cold Temperatures)