Topic
113g Inverted canister stove
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Home › Forums › Gear Forums › Make Your Own Gear › 113g Inverted canister stove
- This topic has 100 replies, 10 voices, and was last updated 4 years, 4 months ago by
 Jerry Adams.
Jerry Adams.
-
AuthorPosts
-
Aug 29, 2020 at 6:39 pm #3673782
Yes
But upright stoves can be used in cold Temps just fine with those techniques, which aren’t that difficult, and yeah, BYOS
Aug 29, 2020 at 7:12 pm #3673788I completely emptied one of the Boss canisters. I then added 1 ounce of propane by weighing the canister on my postal scale. The temperature outside was 70 degrees when I transfered the propane from the one pound tank to the small Boss canister by inverting the large tank, opened the valve, waited for the scale to register 1 ounce.
With only one ounce of propane in the Boss canister, how much pressure on the inside of the can is caused by the propane boiling off?
Aug 29, 2020 at 8:42 pm #3673801Dan
The actual amount of liquid propane in the canister makes no difference, be it 10 g, 100 g, or 200 g. No difference at all. It’s when you blend the propane with something else, like butane, that the total pressure changes. It is all explained in the article I cited.
I assume you mean 70 F? That’s about 21 C. The vapour pressure of propane at that temp would be about 9.5 atmospheres gauge (ie above ambient).
There are several different equations for the P/T relationship, and they give slightly different results at higher temps. But only slightly.
Cheers
Aug 30, 2020 at 9:25 am #3673846With less propane, if it burst, it would be less catastrophic
Actually, I bet what would happen would be the bottom of the canister would just push out – go from concave to convex. Totally anti climactic
Aug 30, 2020 at 9:43 am #3673852Roger,
PV = nRT
Seems the amount of propane (or the n term for moles of gas) is directly proportional to the pressure.
What am I missing?
Aug 30, 2020 at 2:42 pm #3673917I think that’s the number of moles in the gas
You can have more or less liquid under that gas and it won’t make any difference
Aug 30, 2020 at 2:58 pm #3673926I had to ask google what Roger was talking about. Google said the pressure inside the BOSS canister that contains 97% propane is about 132 pounds per square in (psi).
Thank you very much Roger for your scientific information.
Roger said:
However, putting 100% propane in a canister designed for 60% propane is RISKY. I could imagine a hot day, or in a car in the sun, could take 100% propane above the burst strength of the canister. This would not ‘be good’.
Roger, why make the above comment when you know all along that it makes no difference?this is what you said to me:
Dan
The actual amount of liquid propane in the canister makes no difference, be it 10 g, 100 g, or 200 g. No difference at all.
Aug 30, 2020 at 4:02 pm #3673942Oh dear, where to start?
The actual amount of liquid propane in a canister (as long as it is >0) does NOT change the pressure in the canister. The pressure follows a known curve of pressure vs temperature.

This is what happens when you ‘persuade’ a canister to burst. There is a silly myth that the bottom just everts. The reality is that the crimped seam around the bottom eventually fails and the bottom ‘peels’ off the top, as shown here. This happens very fast, with a big bang. A really big bang.Read our article on exploding canisters:
https://backpackinglight.com/exploding_gas_canisters_the_hazard_of_overheating/
The information is all there.The result is a ball of expanding propane gas. The more there was in the canister, the bigger the ball of course. If there is a flame nearby, the result is even worse: the propane burns with another huge explosion in volume. You can destroy a large solid brick building this way: it is called a ‘Fuel/Air Explosion’ (FAE). Look it up.
‘PV = nRT’ is the GAS law. It does NOT apply to a liquid. What happens inside a canister is that the liquid fuel boils until the pressure is high enough that no more can boil off. Equilibrium is reached. Take some gas out and more liquid boils. That is how an upright canister stove works.
Cheers
Aug 30, 2020 at 4:13 pm #3673943Thank you.
Aug 30, 2020 at 4:55 pm #3673953I thought the bottom would just evert
I remember now that article
Still, probably not an explosive fireball
I think if you put pure propane in a canister it would make sense to test it at home. Put it at a temperature that exceeds anything you’ll experience in the field.
Aug 30, 2020 at 5:27 pm #3673962Neither I nor BPL can accept any responsibility for any accidents anyone has while playing with canisters.
Trust me, even without a flame the explosion WAS huge. I did not test it with a flame. I am not (that) stupid.
IF you want to test it at home, do it outdoors with NO flame around. I used an electric hotplate to warm a pot of water in which the canister was sitting. The rate of temperature rise was low. The canister went up, and it was a long while before it hit the ground. Me, I was out of sight behind a steel wall the whole time.
It always amazes me that any company managed to persuade their lawyers that they should get into this business. We did have a big argument with MSR and their lawyers over the CO safety of the Reactor stove at one stage. You may notice the multiple warnings they have about CO all over the Reactor.
Cheers
Aug 30, 2020 at 6:43 pm #3673969Roger said:
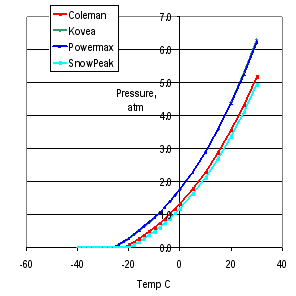
Now, my testing (in the article) showed that a single canister did get up to 100C or >30 atm before exploding, but that is NOT guaranteed for any canister. Yes, they build them a bit over-spec – so they have zero failures at all times. But that was a sample of one, and it was undamaged at the start.
Do you feel lucky?
100C equals 212 degreesF That is way way way beyond any temperature a backpacking canister will ever get in the back seat of your car or backpack.
The BOSS canister was made to handle PROPANE. And yes, they are manufactured over-spec like Roger points out. They have to meet DoT requirements. A little added protection never hurts;)
The company that fills the BOSS canisters submitted a Material Safety Data Sheet that lists the contents. Did they make a mistake on the pressures that Roger read….dunno. Did Roger make a mistake…..dunno.
Aug 30, 2020 at 8:45 pm #3673981The BOSS canister was made to handle PROPANE.
I have to disagree here.
If the density quoted in the BOSS MSDS is correct, then it was made to handle something like 60%propane/40%butane. There would be some serious legal penalties for errors in an MSDS.I am not questioning whether the propane fraction in the BOSS canisters is higher than we get in our ordinary canisters: it probably is. I doubt very much that the DoT would care about them calling it a ‘propane’ canister if the mix has some butane in it: that is not their worry. It is just that I don’t think the canister is rated for 100% propane, and never mind the label.
Stay Safe!
Aug 31, 2020 at 6:12 pm #3674108I disagree also. We can do that :-)
When the butane stops evaporating under -3C the propane takes over and presurizes the canister. The canister is manufactured to resist that pressure. The hand held torch is used in extreme environments to do repairs. The canister absolutely has to be manufactured to sustain the propanes high vapour pressure.
Aug 31, 2020 at 6:34 pm #3674112<p style=”text-align: left;”>And your data to support that statement is …?</p>
Aug 31, 2020 at 7:04 pm #3674121

 Aug 31, 2020 at 9:14 pm #3674138
Aug 31, 2020 at 9:14 pm #3674138When the butane stops evaporating under -3C the propane takes over and presurizes the canister. The canister is manufactured to resist that pressure. The hand held torch is used in extreme environments to do repairs. The canister absolutely has to be manufactured to sustain the propanes high vapour pressure.
Unfortunately, that is NOT how things work.
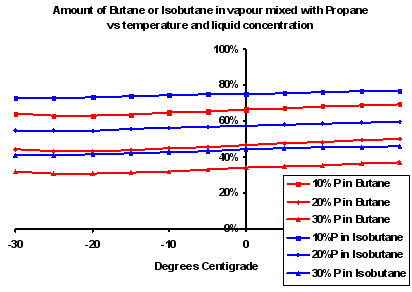
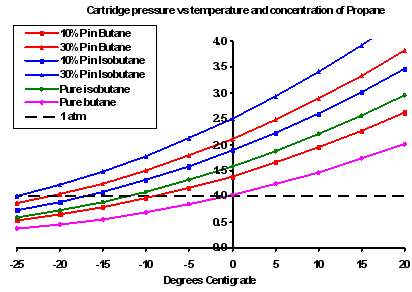
When the butane gets below 0 C (n-butane) or -11 C (iso-butane), it doe not actually stop evaporating. Yes, it stops boiling, but the two are different things. Butane will continue to evaporate down to seriously low temperatures – just not very much. OK, you won’t get any butane gas out of a butane canister at really cold temps.The total pressure inside the canister is the sum of the partial pressures of both the propane and the butane. This fact is apparently not intuitively obvious. However, by the time the fuel is significantly below 0 C, the partial pressure of propane is also much lower. At 0 C the vapour pressure of pure propane is about 1/3 of the pressure inside a new canister of 30%propane/70% butane on a 50 C day.
So yes, the canister has to be able to withstand the pressure of pure propane – at 0 C. BUT that pressure is well below what the canister has to withstand when new at 50 C.
It is all explained in our Exploding Canisters article.
Cheers
Sep 1, 2020 at 7:20 pm #3674359Roger, this is the thread you made me believe I was putting 100% propane in the BOSS canister. Read what I said about 98% and then read your response:

 Sep 1, 2020 at 8:32 pm #3674384
Sep 1, 2020 at 8:32 pm #3674384I cannot understand how you got the 100% idea from what I wrote.
I wrote that I thought the BOSS canisters contain 60% propane and 40% butane, based on the MSDS density.
I then added a safety warning about putting straight propane in the BOSS canister.Cheers
Sep 1, 2020 at 8:58 pm #3674393Here’s more mentioning of 100% propane. 1 pound Coleman tanks don’t contain 100% propane:
 Sep 1, 2020 at 9:09 pm #3674397
Sep 1, 2020 at 9:09 pm #3674397What point you’re trying to make Dan? If there was confusion or lack of clarity regarding what is actually in a bottle of “propane,” hasn’t that been sufficiently cleared up?
Do you think Roger has deliberately deceived or carelessly misled you somehow? Are you asking him to admit it, and/or apologize?
Sep 1, 2020 at 9:17 pm #3674398Do you think Roger has deliberately deceived or carelessly misled you somehow? Are you asking him to admit it, and/or apologize?
Absolutely not.
Sep 1, 2020 at 9:55 pm #3674401Boring.
Sep 1, 2020 at 10:14 pm #3674402My winter kit using the Volcano stove and small BOSS canister of Propane fuel. Stove operates flawlessly in 7mph winds without windscreen/sheild. Not recommended for use inside tents unless your feeling lucky.
 Sep 2, 2020 at 7:52 am #3674423
Sep 2, 2020 at 7:52 am #3674423I think these several threads had good info
Sometimes people misinterpret each other, thread goes sideways on irrelevant issues, not to worry…
Sometimes someone says something that’s wrong or off track, eventually things get to a better place
In the end, there are ideas expressed. People can try different ideas that apply to them and find out what works for them.
-
AuthorPosts
- You must be logged in to reply to this topic.
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Our Community Posts are Moderated
Backpacking Light community posts are moderated and here to foster helpful and positive discussions about lightweight backpacking. Please be mindful of our values and boundaries and review our Community Guidelines prior to posting.
Get the Newsletter
Gear Research & Discovery Tools
- Browse our curated Gear Shop
- See the latest Gear Deals and Sales
- Our Recommendations
- Search for Gear on Sale with the Gear Finder
- Used Gear Swap
- Member Gear Reviews and BPL Gear Review Articles
- Browse by Gear Type or Brand.