Topic
Pure propane inside a 70/30 canister – maximum payload ?
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Home › Forums › Gear Forums › Gear (General) › Pure propane inside a 70/30 canister – maximum payload ?
- This topic has 23 replies, 5 voices, and was last updated 3 years, 2 months ago by
 Roger Caffin.
Roger Caffin.
-
AuthorPosts
-
Oct 31, 2021 at 4:14 am #3731064
What would be the maximum pure propane payload in a 70/30 butane / propane mix canister ?
30% seems intuitively possible, but because there will be more empty room it seems that more is possible.
How to compute this to keep the canister in the same pressure safety limits ?
Oct 31, 2021 at 7:20 am #3731067Oliveir A, Well, there are calculations and other calculations. I am not sure what they use on canisters. 70/30 is in percent, soo, simply multiplying thru with 30% on an 220gm canister, yields 66gm of propane in a can. In chemistry, they add up molecular weight (moles) at standard pressure/temperature(STP) and multiply through with 0.3 to get the total amount. There are other PERCENTAGES possible (say ratio of pressures) depending on what you are looking at, but I suspect that the first calculation is close enough for what you want.
I’m not sure 100% propane is possible except at frigid temps (ie, <32F or 0C.) Pressure increases with temp increases, or, the corollary, pressure decreases with cooling. Generally the boiling point of propane is much lower than the butane family (n-butane, isobutane.) So, keeping the temp at say -43F (the boiling point of propane) you could load it up with 100% propane with no problems. Buy, of course, it would not power a stove at that temp, either. Most canister fuels started with using waste butane gas from refineries. This was a mix of all sorts of gasses, actually, but generally n-butane & isobutane were the primary ingredients. By isolating isobutane, more “heat” could be packed into the same can,, ie more burnable molecules at less pressure. Adding propane adds more heat, but also the corresponding pressure increases, since the propane fraction in the can is increased. So, the same specifications on a can will allow a 75/25 mix of isobutane/propane. 70/30 is close to the maximum. This is about the limit for cans with a safety margin. Why? Because more propane increases the pressure inside the can. Adding propane increases the cold weather performance, also. Propane will tend to fractionate (a type of distillation) into lower percentages when in use, since it will boil (increasing pressure) at a lower temp than butane. You start running into gas laws, and other calculations, of course. As an example, there are stories of half full cans that will not burn when connected to a stove at low temps. The propane has been previously burned off when winter hiking/camping, hence little is left to power a “topper” stove.
So it is a curve of temp/pressure that will determine when you can use increasingly pure propane. As a rule of thumb, I would use no more than a 50/50 mix at any normal winter hiking temp. This will give you some margin of safety at 32F/0C, less with any increase in temp (say carrying a can in a car to/from a trail head.) Cans will only contain so much. pressure Letting them warm up to say 81F/27C, could potentially exceed the safety standard on a can, and have it explode in your backpack I believe Roger C examined this in much more detail than I have. Perhaps he will chime in. Unfortunatly my chemistry is 50 years old and I don’t remember the exact calculations involved.
It doesn’t really matter if there is more room in a can. DO NOT FILL IT UP. The space provides a buffer between liquid (propane/butane, etc) and gas. Common acetylene cylinders use a butane mix to absorb the more reactive acetylene molecules, thus lowering the pressure in a tank. They still only add just so much and leave about 15-20% empty space. Think of boiling water in a sealed container. High pressure steam will explode a container IFF there is no room for thermal expansion! Even water bottles should not be filled to 100% when hiking, they can produce leaks because of the temp/pressure. (Freezing a beer in a closed container will usually result in a mess.)
Anyway, I would not use 100% propane in a standard canister. Again, 60/40 or so is about the most I would risk. I believe an old, obsolete canister design based on a smaller cylinder was used unsuccessfully 20-30 years ago at 60/40. But this was designed around the shortfalls of a standard canister.Oct 31, 2021 at 8:23 am #3731069This has been discussed in previous links
You can put 8 ounces of pure propane in an 8 ounce canister.
The problem is you can’t let the canister exceed some temperature, like 70 F?, or the canister can burst. Propane has a higher pressure at the same temperature as butane. Which goes along with it’s lower boiling temperature.
Oct 31, 2021 at 8:38 am #3731071Thanks James for all those details. My question was just about the filling limit of a 70/30 canister with pure propane. More for curiosity.
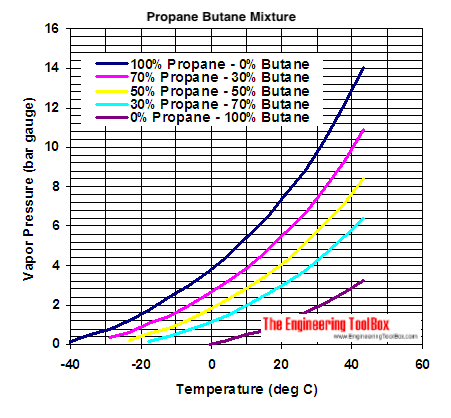
When we watch the propane / butane pressure curves for different mixes, we can clearly see that pure propane has a higher pressure :

But when a canister become empty, the pressure reduce regardless the mix.
This is why intuitively we could think that it should be possible to fill a 70/30 canister at 30% with pure propane. For example, filling a 100g empty canister with 30g of propane.
Is it safe to do that ?
Or in other terms, at 40°C for example, witch canister would have the higher pressure :
– a 100g canister filled with 30g of propane ?
– a 100g canister filled with 30g of propane plus 70g of butane ?
Pressure limit of a EN417 canister seems to be 6.6 bars.
Oct 31, 2021 at 9:51 am #3731074You said: This is why intuitively we could think that it should be possible to fill a 70/30 canister at 30% with pure propane.
No. A mix will respond differently than a pure substance. In the butane/propane mixes, the propane dissolves into the butane, reducing it’s pressure even more..
Well, you get into a lot of chemistry about mixes and gas pressures with various mixes in a closed container. Basically that is what a can is, a closed container. Think of pressure as the force exerted on the can by the type of molecule involved. So, a mix will exert the force of say 1/3 propane and 2/3 butane. This isn’t real accurate but close enough for the example. By increasing the pressure (by adding propane,) the can needs to hold more and more pressure. By filling a can fully, you introduce all sorts of thermal expansion, besides.Never fill a can fully!
In the above example, pure propane vs a 70/30 mix, you will find that the mix will have a lower pressure, despite the same quantity of propane. Now the straight multiplication I gave as an example will break down to molar pressures and other things my 50year old chemistry taught and I forget (absorption, pressure per gas, etc,) but, suffice it to say that the absorption (by the butane) of the propane will result in a lower static pressure. The surface also only comprises the 2/3 mix of butane. Soo, 2/3 of the molecules will boil off as butane with only 1/3 boiling off as propane, as an inaccurate example. This is roughly the same as my acetylene tank example, above. Because the absorption of the propane at the surface is so much higher than the butane’s release of its molecules, it will actually reduce the can’s pressure. 2/3 pressure is contributed by the butane, 1/3 third of the pressure is the propane’s, to quote my previous, wildly inaccurate example. There simply isn’t enough room at the surface to boil off only propane at it’s higher pressure.
This is one of the reasons that you need a surface in the can (ie leave the can 25% empty,) not just for thermal expansion. Of course, it also leaves space to avoid the hydraulics of thermal expansion..
(Hydraulics says that a full can will expand by the expansion of the liquid, often at a much higher pressure than the actual surface pressure, assumed to be extremely large. Liquids and solids cannot be compressed (again, not quite true.) Soo, you end up with a huge pressure on the can from both. Filling a can fully sounds like a bad idea to me.)
In a non-mixed system, this is the full pressure of the gas, propane in this case. Again, mixes respond differently than a pure substance. We will ignore the boundary cases because you will never hit them. (Unless you head out at -43F.) But 100% of the pressure is from the propane…much higher than a mix.Oct 31, 2021 at 4:22 pm #3731120If you can go up to 6.6 bars without the canister bursting
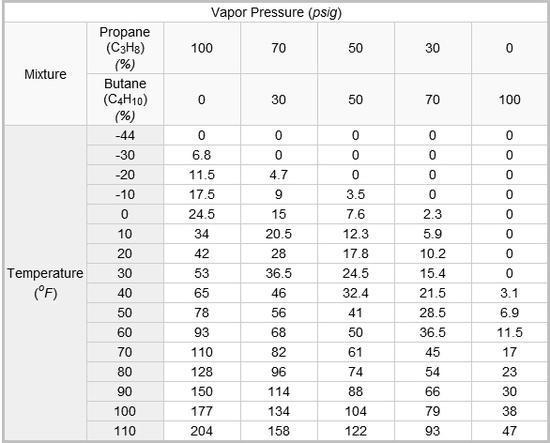
from your graph, for pure propane, at about 17 C (63 F) it produces about 6.6 bar.
if you keep the canister below 63 F, you can put pure propane in it without bursting
For butane or propane you want to avoid the situation where you completely fill the canister, then warm it up. It will expand and burst the canister. That’s why they don’t fill them completely. You don’t want to put more butane or propane in it than that amount. Like James said.
You want to weigh the canister when it’s full. Then, if you refill, weigh it and make sure you don’t exceed the original weight. (You could just assume that 8 ounce containers weigh 13 ounces when full, 4 ounce containers weigh 7 ounces. There’s enough safety margin this should be close enough).
The density of propane is 16% less than butane so you should probably put in 16% less weight of propane. So, put 7 ounces of propane in an 8 ounce canister, so final weight shouldn’t exceed 12 ounces, 4 ounce canister – 6.5 ounces
I’ve probably made at least one math mistake : )
Oct 31, 2021 at 4:37 pm #3731123Thanks, Jerry. I would object to a 63F maximum. I would go with a 50/50 mix and have the damn thing a bit safer. Even at 30C/86F is still not much margin for 3 season hiking.
Oct 31, 2021 at 5:56 pm #3731126Or in other terms, at 40°C for example, witch canister would have the higher pressure :
– a 100g canister filled with 30g of propane ?
– a 100g canister filled with 30g of propane plus 70g of butane ?
Pressure limit of a EN417 canister seems to be 6.6 bars.James has covered a lot of this, but just to make things very clear:
If you FILL a canister with any gas mix, it will burst when the temperature rises. Not because of the increase in vapour pressure, but because of the increase in volume due to thermal expansion of the LIQUID.
That is why you NEVER really FILL a canister.Now, which of the two cases above would have the higher pressure if we assume the canister has not been really filled with liquid?
The pure propane case would have the higher pressure.
Things do get a little complicated when playing around with gas mixtures, again as James was explaining. In very simple terms, the lower pressure butane dilutes the higher pressure propane.There is another good point in all this: which canister would have the higher pressure, assuming neither case was ‘full’:
A canister with 30 g of propane
A canister with 60 g of propane?The answer is they would both have the same internal vapour pressure. The amount of liquid present does not affect the vapour pressure.; only the composition matters.
For about the same reason, having a blotting paper liner in the canister (aka Primus Winter Gas can) does not have any effect on the performance of the canister. This was discussed in https://backpackinglight.com/primus-winter-gas-review/ .
The only effect is to significantly raise the price, for which you get nothing extra.Cheers
Oct 31, 2021 at 10:09 pm #3731133Jerry’s right – butane is 1.16 times the density of propane so you shouldn’t put in the same total weight of pure propane as 30/70 propane/butane.
Pure Butane: 573g/l
Pure Propane: 492g/l
30/70 mix: 546g/l
So for every 100 grams of 30/70 was originally in there, you should only refill with 95 grams of propane to have the same liquid volume (hence the same headspace) volume at 20C.
And then there’s the pesky fact that the thermal expansion rate of propane is higher that than of butane. So we want even less weight in the canister to provide the same ability to expand (although I expect the failure mode won’t be from completely filling with hot expanded liquid, but will be due to propane’s vapor pressure). Anyway, to be safe, I’d refill only to 90% of the original fuel weight.
Someone ought to repeat Roger’s experiment taking a canister to failure (at 98C / 208F if I recall), but with pure propane. Please someone else take that on, or the guy on 13 snow-covered acres will have to do it, far from the house, when my wife isn’t around.
But, rereading the OP: ONLY KEEPING THE PROPANE FRACTION AT OR BELOW 30% WILL MAINTAIN THE SAME SAFETY MARGINS. All higher propane fractions will reduce safety margins and reduce the safe operating and storage temperatures, regardless of fill volume/weight.
Nov 1, 2021 at 4:43 am #3731143There is another good point in all this: which canister would have the higher pressure, assuming neither case was ‘full’:
A canister with 30 g of propane
A canister with 60 g of propane?The answer is they would both have the same internal vapour pressure. The amount of liquid present does not affect the vapour pressure.; only the composition matters.
Thanks Roger and all who answered, but i still have a couple things i’m not sure about :
1) When a canister become empty, it’s pressure reduce, but when ? Only when there is no more liquid gas, when there is only vapor gaz remaining in the canister ? If this is the case, this mean that for a propane butane mix, there will be mainly two pressure levels in the canister (when used in non inverted position), a higher one until the more volatile propane gas has ended, a second pressure level after corresponding to the vapor pressure of butane . A third decaying pressure level when there is no more liquid gas at all. Am i right here ?
2) The vapor pressure is higher for pure propane, this mean that even with a canister filled only at 1/3 of full capacity, the safety margin is reduced : the pressure will be higher than the EN417 6.6 bars limit as soon as temperature will rise above 17°C, regardless the remaining capacity in the canister. Am i right here ?
3) The canisters are guaranteed at 6.6 bars, this is the minimum requirement. This limit is reached with propane at around 17°C. Even if liquid expansion can occur (enough empty room in the canister) the pressure can easily reach 16 bars or more at 50°C. Seems like pure propane is never absolutely safe in a camping canister, even if the 6.6 bars limit is probably a very pessimist limit. Here we had bad stories some years ago with butane canisters that did not resist to summer sun.
The conclusion if i’m right is that is is never possible to put pure propane in a camping EN417 canister manufactured for 70/30 mixes and stay fully safe up to 50°C, except if the quantity is very low. Very low would mean that the quantity is low enough to not have liquid gas at all in the canister. The weight of this “safe” pure propane payload would be approximately the weight of propane vapor corresponding to the volume of the canister. For example a rapid calculus for a 450g canister (around 800ml volume) : the weight of fully safe pure propane payload would be around 1.4 g :)
I took a density of 1.8 Kg/m3 to get this result. This is the density of propane gas at 25°C.
Nov 1, 2021 at 8:24 am #3731154I was thinking of that David has mentioned that he uses pure propane in Alaska when it’s cold. No problem staying below 17 C/63 F.
But, as I’ve mentioned before, if you take David’s two ideas – torch lighter (1.3 ounces) and applying it to the side of the canister down below where the fuel is – that is the easiest way to run your canister stove when it’s cold. In my experience.
Nov 1, 2021 at 8:25 am #3731155You have to put your torch lighter in your pocket or under your armpit to warm it up so it will work.
Nov 1, 2021 at 8:32 am #3731156Oliver,
Number 1
No, The pressure does not reduce at the same temp. Often, we are fooled into that assumption because the canister will also COOL as you boil off what fuel is in there. Temperature and pressure are related. As the temp goes up, so does the pressure. Conversely, as the temp goes down, so does the pressure. As long as you have liuid in the can to boil, pressure remains the same at the same temp. Generally, we think of a mixed fuel as the same as a single fuel. Soo, the second part of this makes no sense. There are not two different pressure waves. Well, at the very empty or very full states, you might run into it. Think of hydraulic pressure instead of vapor pressure, or, just plane empty. There is no “decaying” pressure except with temperature as long as you have liquid fuel in the can.Number 2
Yes, you are correct.Number 3
That is my opinion. There is no safe condition for pure propane with existing cans. However, with a format change, it could be possible. A longish tubed shaped can will hold more pressure than the current cans. Look at a current propane cylinder. But, these are invariably heavier than current cans.Your conclusions::
Well, given your initial premise, I wonder how you arrived at the correct conclusion. Yes, you are correct, mostly. But, even with that, there are caveats. You didn’t say what pressure. If you start with a 6.6bar of pressure and fill to that at 50C, then you may NOT be safe at 60C or anything above the 50C. Again, you are better off with a more’r’less 60/40 mix of butane/propane at <32F and take the slight loss in fuel efficiency, in the interests of safety. This will give you about a 2x safety margin for warming, you will get a lot more propane in the can (because of mutual disolving) and a higher safety margin..
Nov 1, 2021 at 9:06 am #3731212I just fill my canister with enough fuel for a particular trip. If I’m going for two nights, maybe I’ll put 3 or 4 nights worth of fuel (extra margin so I don’t run out, and it really slows down when it has less than one ounce left). If I’m going for 6 nights I’ll put 7 or 8 days worth, which would be close to filling to capacity. Maybe 7.5 ounces in an 8 ounce canister – no need to risk over filling.
Nov 1, 2021 at 11:23 am #37312221. Yes, pressure will be somewhat constant as long as there is liquid remaining. “Somewhat” because the propane fraction decreases throughout canister life and because as JamesM points out, vapor withdrawal cools a canister during use and that reduces vapor pressure. No, it isn’t propane vapor then butane vapor – it’s a continuum with more than 30% propane in the vapor at the start and less at the end. So (if kept at a constant temperature), pressure would slowly decline through its use until there was no liquid left and then it would quickly and linearly go to 1 atmosphere of pressure. Below 1 atm, there can be no flow out of the canister.
2. Yes. Any liquid pure propane and it’ll be at propane’s vapor pressure which will reduce safety margins.
3. The canisters are rated to 6.6 bars, but we know they can handle a lot more, based on Roger’s test to failure. How much more? That may vary from one size and source to another. How much safety margin are you willing to give up? One might think, “I’m on a snow-camping trip – it can’t get above 5C the whole trip.” but forget that they’re staying in a USFS cabin with a wood stove or that their vehicle is heated (quite a bit near the heater outlet) or consider the radiant heat from the burner reflected to the canister or that a pot of hot ramen spilled onto the canister could quickly rise its temperature a lot.
Yes, I also calculate about 1.5 grams of propane vapor only in a large canister AT ONE ATMOSPHERIC PRESSURE. Not enough to boil a single cup of water. But we can bring it up to 7 atmospheres at 50C so that allows for about 9 grams of propane vapor (no liquid) in the canister and now you could boil one pint of water.
Nov 1, 2021 at 11:38 am #3731230So in the end 9g of pure propane would be safely kept in a 450g canister ! Not so bad if this can save a life :) :)
Nov 1, 2021 at 2:08 pm #3731239The conclusion if i’m right is that is is never possible to put pure propane in a camping EN417 canister manufactured for 70/30 mixes and stay fully safe up to 50°C, except if the quantity is very low.
Correct.
David can get away with it in Alaska in the winter. Reliably cold up there. Pluses and minuses!But all of this ignores the question of whether you need to put 100% propane in a canister. In real life, you do not.
My canister sits in my pack next to my water bottle, against my back. The water does not freeze while we are traveling, so I know the canister stays >0 C. Overnight the canister lives under the foot of my quilt, which (I assure you) keeps it >0 C as well. Plus I use an inverted canister stove most of the time, so the canister does not cool down while I am cooking for two.
While it might be nice to be able to use LPG from a 10 kg tank as a very cheap refill, it simply is not necessary in practice. In fact, in summer time I often use ‘butane’ from those cheap fly-spray cans as a refill, as the canister never gets anywhere near 0 C.
Cheers
Nov 1, 2021 at 3:19 pm #3731244Yes in practice pure propane is not so useful for backpacking. But could be useful when using a powerful dual burner stove near 0°C in the camp. In this case using a regular propane tank, refillable or not, seems to be a good solution. This is the solution i did choose : a small 1.6 Kg refillable propane tank for my dual burner stove.
My questions were mainly for curiosity and understanding things that were a bit obscure for me. Gas is not so simple. I think that i prefer nuclear fission, this is simpler. Perhaps for my next stove :)
Nov 1, 2021 at 4:08 pm #3731245Roger got explicit on a point that was rattling around in my head: Why?
Instead, just
– Use a liquid feed butane/propane canister stove like Roger’s as long as you’re above the mixture’s boiling point and just need to avoid evaporative cooling.
– A standard canister is good down to -25F/-32C with a Moulder Strip, as I’ve demonstrated. Other tricks (IR reflectors, armpits, warm water) also extend the range of standard mixtures.
– Asian grocery store vertical butane canisters give you moderately cheap summer fuel.
– It’s rare for me to be UL in winter. I’m dragging a sled across a frozen lake (which 19/20 times are perfectly flat and that one time, post-quake, in 2016, the lake wasn’t far from flat). Or car camping. Or just using one of my stoves in the backyard. In all of those cases, the dark-green 1-pound propane in 1-pound of steel “Coleman” propane cylinders are fine. And super easy, cheap and safe to refill from 20-pound BBQ tanks.
Nov 1, 2021 at 4:38 pm #3731251MSR Isopro or other fuel that’s isobutane is good down to 20 F. Maybe 16 F if you don’t mind it being slow. With an upright stove.
I can’t imagine anyone trying to camp colder than that : )
Nov 1, 2021 at 4:41 pm #3731252I’ve noticed that I operate butane as much as 5 F colder than others recommend. I don’t know what that’s about because I’m a bit anal about looking at my thermometer. And making sure it’s calibrated.
Nov 1, 2021 at 4:58 pm #3731253Jerry, my first winter in Alaska – 1998/99 – I concluded that the bottom of my fun meter was -15F. I can function at work and survive at lower temps, but I’m never having fun when it’s colder that that. The dog agreed – she wasn’t sad to turn around and head home.
Being increasingly resigned to this falling to me to field test and starting with this chart:

I see, for instance, 100% propane at 60F has the same v.p. as 30% propane at 110F (93 psig). Several other data points also show 100% propane have the same v.p. as our backpacking mixtures at a temperature 50F cooler. So if Roger got a mixture to explode a backpacking canister at 98C/208F, it seems I should expect failure around 150-160F.
I’m imaging a bucket of water, with a 1500-watt electric element in it. I could 1) set up a thermocouple on a long cord and data-log it manually, 2) install a dial thermometer and data-log (and document the failure) with a GoPro or 3) time the heating of 1-gallon of water from 50F to 160F in person (no canister) and then just listen for the boom with a stopwatch.
I’m leaning towards the GoPro, maybe after establishing a time scale sans canister.
Nov 1, 2021 at 5:14 pm #3731254if there’s no video then it didn’t happen
not that I want to encourage people to take video of dangerous things. Or of things that damage other people’s stuff.
Nov 1, 2021 at 9:41 pm #3731276I’m imaging a bucket of water, with a 1500-watt electric element in it. I could 1) set up a thermocouple on a long cord and data-log it manually,
I used a lab hot plate and a tin can of water. The hot plate was robust enough, and the tin can – well, it was free.You could use an LM35 sensor and a voltmeter – and a very long wire!
If you are going to data log manually, having two people is much easier. One person holds the watch and the voltmeter and calls out the readings at 10 second intervals. The 2nd person writes.Mind you, seeing the bang on a video could be … cute.
Cheers
-
AuthorPosts
- You must be logged in to reply to this topic.
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Our Community Posts are Moderated
Backpacking Light community posts are moderated and here to foster helpful and positive discussions about lightweight backpacking. Please be mindful of our values and boundaries and review our Community Guidelines prior to posting.
Get the Newsletter
Gear Research & Discovery Tools
- Browse our curated Gear Shop
- See the latest Gear Deals and Sales
- Our Recommendations
- Search for Gear on Sale with the Gear Finder
- Used Gear Swap
- Member Gear Reviews and BPL Gear Review Articles
- Browse by Gear Type or Brand.