Topic
Air-Activated Heating/Cooking Systems
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Home › Forums › Gear Forums › Make Your Own Gear › Air-Activated Heating/Cooking Systems
- This topic has 16 replies, 11 voices, and was last updated 5 years, 11 months ago by
 Diane “Piper” Soini.
Diane “Piper” Soini.
-
AuthorPosts
-
Jan 25, 2019 at 6:27 am #3574958
I have been performing a fair bit of research into ‘flameless’ cooking systems in recent months. There are various ready made solutions out there including:
1. Trekmates Flameless Cooking Flask.
2. Barocook.
3. Yellowstone Flameless Cookset.
4. Yabul Cook.
5. Various suppliers of MRE meals such as Mountain House that supply ready prepared meals packaged with water activated exothermic heating pads.For each of these systems the exothermic reaction to create heat is water activated satchels. These systems create enough heat to reheat a meal, not enough heat to actually cook a meal.
<span style=”text-decoration: underline;”>AIR ACTIVATED HEATING SYSTEMS</span>
In more recent times there are now air-activated heating strips. They are lighter, more practical to us, and can generate greater heat such that meals can be actually cooked as opposed to simply reheated [reference: https://www.youtube.com/watch?v=H2oKrGqkf2o].Ultimately I would hope that companies such as Exothermix (https://www.exothermix.com/) could create either circular heat pads matching pot diameters, or perhaps longer strips length equivalent to the circumference of the pots. Pads could be readily adhered to the underside of your titanium cooking pot, peel of the strip and start to cook!
I would challenge anyone to find a safer, lighter, more convenient cooking method out there to throw into the backpack.
<span style=”text-decoration: underline;”>QUERY</span>
a. Are there any members/users out there that have had any experience with such heating systems?
b. Has anyone been able to find a source that sells ‘air activated MRE heating pads’?Jan 25, 2019 at 2:16 pm #3574972What is the weight of one expended “unit” to heat 16 ounces of 50F water to 150F?
Jan 25, 2019 at 2:48 pm #3574976if it’s iron oxide, can you dispose on the ground if it’s not visible to other people?
Jan 25, 2019 at 2:52 pm #3574977Ah yes. The old “spread your coffee grounds around” debate.
Jan 25, 2019 at 2:57 pm #3574979It doesn’t sound practical for backpacking. At least with a regular stove I have control over burn time and intensity. Without generating garbage every time. And canisters are recyclable.
I would need to use 14-16 to equal the boils out of one 110 canister. No way is that more convenient.
Jan 25, 2019 at 6:00 pm #3575007“I would challenge anyone to find a safer, lighter, more convenient cooking method out there to throw into the backpack.”



 Jan 25, 2019 at 6:02 pm #3575008
Jan 25, 2019 at 6:02 pm #3575008My guess is that these heaters are using calcium oxide as their exothermic material, along with maybe a bit of a neutralizer like sodium bicarbonate to keep corrosion down in the pots. My guess is that iron is not used because of price, and the heat of the exothermic reaction. Calcium oxide is a very cheap, widely used industrial material, and would be fairly easy to package as a powder. So, all the used heating material could be disposed of in the woods easily since it would basically be limestone and a bit of salt. You would still have the packaging materials to consider as carried waste. However, I’d think that you would need more than an ounce of calcium oxide to heat as described, so the heat packets would quickly add up to considerable weight.
Renais
Jan 25, 2019 at 6:17 pm #3575011The “flameless ration heater” inside an MRE contains finely powdered magnesium metal, alloyed with a small amount of iron, and table salt. One water is added, each powder grain, in salt water, becomes a short-circuited galvanic cell – a little battery, shorted to itself until the magnesium has gone to magnesium hydroxide. The FAA doesn’t like them on planes because they generate hydrogen gas is among the easiest to ignite flammable gases.
Here’s are specifications for one brand: 7.5 grams of a powdered magnesium-iron alloy, consisting of 95 % magnesium and 5 % iron by weight, 0.5 grams of salt, in addition to an inner filler and anti-foaming agent. Upon adding 30 milliliters of water, this mixture can heat a 230 gram meal packet by 100 °F in about 10 minutes, releasing approximately 50 kilojoules of heat energy at about 80 watts.
If the 8 grams of chemicals can be contained in 4 grams of packaging, then you get 50kJ / 12 grams = 4 kJ/gram.
Butane’s heat of combustion is 21,400 BTU/lb = 49,700 kJ/kg = 50 kJ/g. But a 100 / 110-gram canister is almost half canister weight, so a net energy content of 25 kJ/g – still 6 times more energy dense than a FRH. A 220/230-gram canister is about 60% fuel so that gives a net of 30 kJ/g or 7.5 times better than the flameless ration heaters.
The big win for the FRHs would seem to be a for an 1-4 night trip with minimal cooking in that you could forego the stove, fuel and possibly a pot. Or somewhere that had a total stove ban in effect. There are places I go like the Aleutian Islands where you’re not supposed to transport fuel on the jet (although there are always alternatives) and while it is less likely a FRH would be noticed than a JetBoil canister, you’re not supposed to fly with either.
Also, you could save time on high-mileage days by activating a FRH and putting it with your meal in a cozy while you hike. Cooking as you hike is not something you can do with an butane, alcohol, or esbit stove.
Jan 25, 2019 at 6:28 pm #3575012There are other schemes for flameless heaters that add water to calcium carbide (as is used in a “miner’s lamp”). This generates acetylene (also something the FAA doesn’t like on planes) and calcium hydroxide (slaked lime). I’ve used carbide lamps while caving:

and before LED lights, I’d even use them on some group backpacking trips as it provided more light than a flashlight (but less than a Coleman lantern) for less weight per lumen-hour. They definitely get hot, even at a very slow water drip rate to continuously generate a small amount of acetylene.Conservation-minded cavers, like me, don’t want you to dump the waste lime in a cave. Buried and stirred with soil in a forested setting, I don’t see a big issue with it. It “sweetens” acidic soils which helps a lot of plants – just not wetland plants that have evolved to compete in acidic soils.
Jan 25, 2019 at 7:40 pm #3575027Heh, I was just looking up carbide lamps. Someone should double check my math, but I think calcium carbide has 20kJ/g. But that doesn’t include the container / gas generator.
Also: soot.
Jan 25, 2019 at 7:41 pm #3575028On a related note, a while back I looked in to instant cold packs for cooling beverages. Also not worth the weight.
Jan 25, 2019 at 10:39 pm #3575070One UL way I can see to cool a beverage – especially one in a aluminum can, would be with a moistened cotton sleeve, in a windy, shady spot in low-humidity conditions. We tried a “desert water bag” of my grandfathers on an Owens Valley family vacation in the 1970s. It worked. It made cool water on a hot day. That tasted like moldy cotton, but it was 50 years old at that point.

I’d estimate you could get within 10F of the dew point. For instance on an 80F, 25% RH day, the dew point is 40F and you could get your beverage down to 50F, which isn’t exactly American beer temperature but is a whole lot better than 80F beer or soda.
Even more UL would be to just maximize radiant cooling at night: Put the beverage containers in a low spot with a wide view of the clear night sky. You can easily get 10F below the night-time low temperature and as much as 20F colder in ideal conditions with a perfect set-up. Then put it in a cozy, wrapped in clothing and your quilt, and it will stay cold for hours.
Jan 26, 2019 at 1:12 am #3575092No need to speculate: Exothermix uses a zinc-oxygen reaction to generate heat, after failing to market a zinc-alkaline battery under the name Rechargeable Battery Corporation.
https://www.exothermix.com/about-us (scroll down to Our History)
Also from https://www.exothermix.com/simple
The Exothermix heating system harnesses the energy (heat) created from the simple reaction of zinc with air; the same reaction that is found in a hearing aid battery.
Several years ago they developed the “CookPak” ration heater for MREs. One of the claimed advantages is that the process does not generate steam or hydrogen gas.
Somewhat lame CBS video demonstration of CookPak vs. MRE heater:

Can’t find any other trace of CookPak heaters.
Exothermix/RBC has about 20 related patents:
https://patents.google.com/?q=heater&assignee=Rechargeable+Battery+Corp
Maybe a chemical engineer can decipher them :-)
— Rex
PS We never drank from those canvas Desert water bags in the 50s/60s – they were for refilling old radiators that routinely overheated. Jackson Browne’s debut album is often (but erroneously) called “Saturate Before Using” because of the cover image:
 Jan 26, 2019 at 3:24 am #3575112
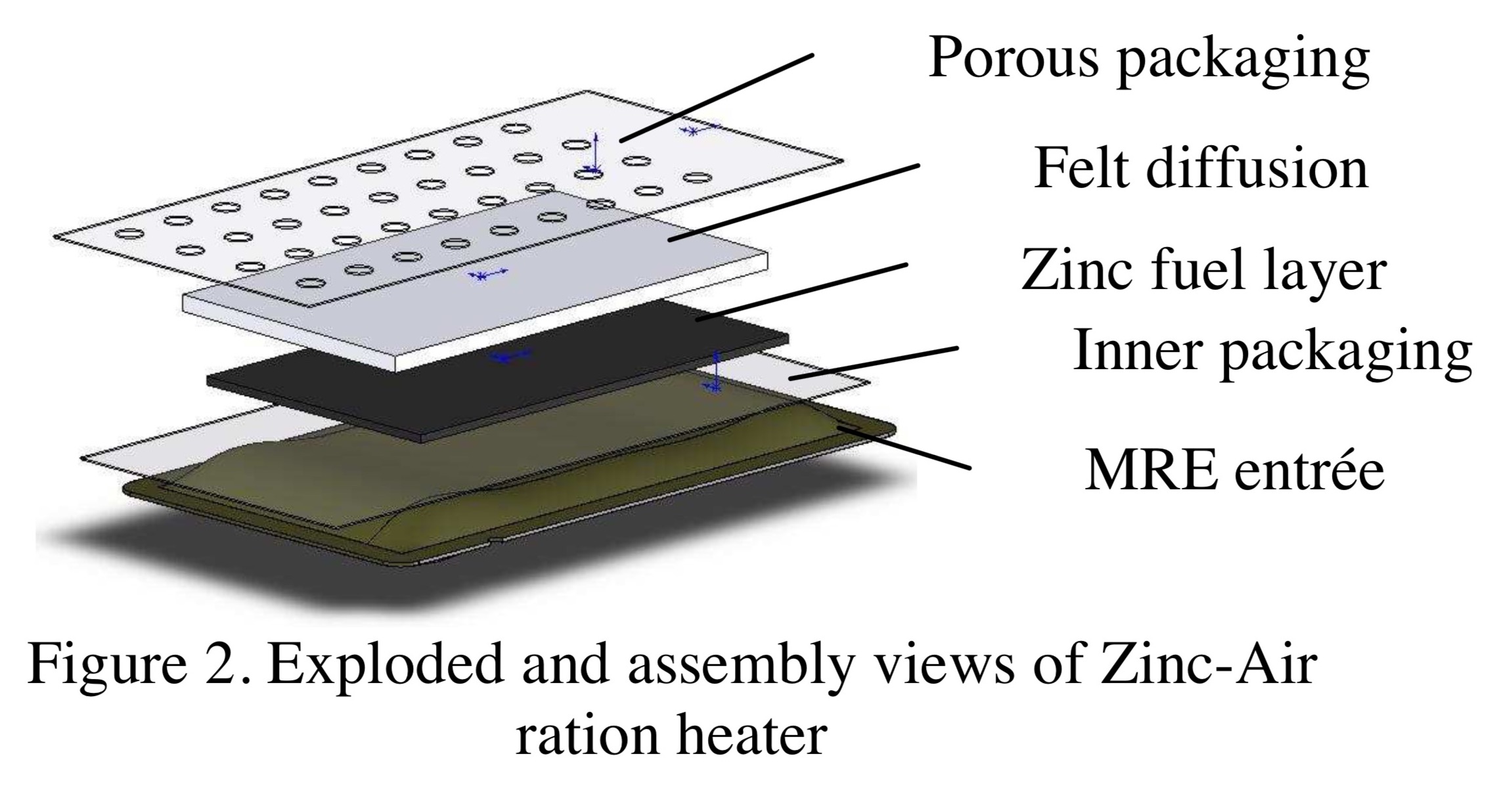
Jan 26, 2019 at 3:24 am #3575112More digging turns up this extremely useful 2008 Army report titled “Air-Activated Ration Heaters,” based on Exothermix/RBC technology:
https://apps.dtic.mil/dtic/tr/fulltext/u2/a504227.pdf
Their main goal was to heat an 8 ounce MRE from 40° F to 140° F within 12 minutes.
They estimate that would take at least 17 grams of raw zinc – plus electrolyte and packaging.
Despite citing many advantages over existing MRE heaters, this technology hasn’t replaced them yet.
Some nice diagrams and photos:


— Rex
Jan 26, 2019 at 6:29 am #3575134Rex: you are +1. The rest of my night is shot on a very fascinating wild goose chase!
I like what David said, there is some potential here for short trips…
Jan 26, 2019 at 9:59 am #3575137Maybe we could adapt some lightweight containers for these,
Feb 10, 2019 at 2:16 am #3577722What about a passive solar cooking system? A bag/cozy that is black on one side, clear on the top side. Put your wet food in a bag, put it in the cozy, put the cozy on top of your pack, enjoy a meal after a day of hiking in the sun. Of course this wouldn’t work everywhere, or maybe most places.
-
AuthorPosts
- You must be logged in to reply to this topic.
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Our Community Posts are Moderated
Backpacking Light community posts are moderated and here to foster helpful and positive discussions about lightweight backpacking. Please be mindful of our values and boundaries and review our Community Guidelines prior to posting.
Get the Newsletter
Gear Research & Discovery Tools
- Browse our curated Gear Shop
- See the latest Gear Deals and Sales
- Our Recommendations
- Search for Gear on Sale with the Gear Finder
- Used Gear Swap
- Member Gear Reviews and BPL Gear Review Articles
- Browse by Gear Type or Brand.