Topic
Jetboil cold weather heat exchanger
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Home › Forums › Gear Forums › Make Your Own Gear › Jetboil cold weather heat exchanger
- This topic has 248 replies, 5 voices, and was last updated 4 years, 10 months ago by
 Jerry Adams.
Jerry Adams.
-
AuthorPosts
-
Feb 26, 2016 at 6:26 pm #3384955
I was using my upright canister at 22 F, and noticed the frost melted in a circle around the stove, several inches out from the stove. It warmed above 32 F. I’m pretty sure it’s radiant heat from the flame and burner and bottom of the pot.
If there was a cylinder of aluminum foil it seems like that could be reflected onto the canister warming it up. Or maybe a cone shape that’s the same diameter as the canister at the bottom and bigger higher. Or just your normal cylindrical windscreen – would need to be only a little bigger than the canister and the inner surface would have to be shiny.
Feb 26, 2016 at 7:00 pm #3384962With a full canister, it only drops 2 F, so you could start at 22 F or higher, and the butane will never get down to that 20 F point, so it will work fine. A full canister will work at a 10 F colder temperature.
As I mentioned earlier, I observed a very quick drop-off in performance of a full 100g canister at 23°F ambient with the standard MSR commercial blend (20 propane/80 isobutane or some such), which suggests a serious disconnect between reality and what is projected to happen according to calculations based upon materials’ properties.
Feb 26, 2016 at 8:28 pm #3384980These recent comments got me thinking about those first tests and the first-gen iterations of the HX strip. And it reminded me how I did not post photos of those setups, which is obviously uncharacteristic of me in this thread.
So, for the sake of the archives and for posteriority, here are the first two, made and tested on 12/4/2014. The first one failed when the solder melted (duh), but the second one provided the encouragement to continue.

 Feb 27, 2016 at 8:03 am #3385025
Feb 27, 2016 at 8:03 am #3385025I attached a silicone temperature sensor to a full and 1/8 full 220 g canister and measured the temperature drop of the 1/8 full at 12 F. The full canister dropped 2 F. This is half as much as theory and there were a couple problems but now it’s too warm to re-measure, maybe winter not over though?
I was just guessing about other sizes but the heat capacity of the butane seems like the most important factor, so 100 g would be the about same as a half full 220 g so maybe it would drop about 8 F.
Feb 27, 2016 at 2:02 pm #3385098Anonymous
InactivePerhaps this is a daft question, but i have no experience with upright canister stoves. If you make it over sized, can you use a Caldera type conical wind screen (wider at base/narrower at top) with these safely? If so, would it help to keep the canister warm enough to function efficiently in colder temps?
Feb 27, 2016 at 2:43 pm #3385103I’ve thought about that too, Justin. The thing about cones is that it gets pretty warm inside, sort of like an oven. That’s why they work so efficiently with alcohol and solid fuel tabs (that, and they also are superb wind screens). Now, is there a way to ensure perfect ventilation to allow just the right amount of heat to escape so as to not over heat the canister? Maybe so, but I’d rather that Bob did this experimentation, and not me. I have always hated being around things that blow up. You know that Roger is having bad dreams right now, sensing that we are even discussing this concept.
A cone would be a bit heavier than a simple Moulder Strip setup, and it is cumbersome and bulky to pack. I think that I can speak for Bob here as well, as we both have the copper strip/cozy thing pretty well fine tuned now, and there’s little reason for either of us to try to find a better way to warm up a canister. This winter I have played with the 3 main concepts–filling the concave canister bottom with warmish water and sealing it with one of those cookie cup lids that I showed you guys, a simple hand warmer under the canister, and the Moulder Strip/cozy. All 3 techniques work pretty well, although the water thing requires that you redo it from time to time as the water cools, and the hand warmer isn’t too effective below + 10* F (I’ve found that it raises the temperature 20* F above ambient). The copper strip works well in the coldest weather that I would hope to be camping in. If things get much below zero F, I’m bailing and checking into a Holiday Inn Express.
Feb 27, 2016 at 4:53 pm #3385129yeah, exactly
with a canister stove you need a windscreen regardless, so if you make it the right shape it should heat up the canister a good bit. And weigh just a couple ounces.
not that this is better than a “Moulder strip” or and inverted canister – many different techniques
Feb 27, 2016 at 5:00 pm #3385134I attached a silicone temperature sensor to a full and 1/8 full 220 g canister and measured the temperature drop of the 1/8 full at 12 F. The full canister dropped 2 F. This is half as much as theory and there were a couple problems but now it’s too warm to re-measure, maybe winter not over though?
After messing with this stuff a lot I’ve come to realize that even the simple question of where to put the temperature probe does not have an easy answer. Temperatures vary widely at different locations on the canister because of stray radiant/convective heat from the burner and pot; stoves themselves conduct heat to the canister (BRS-3000T very significantly); even a slight breeze can affect temperature significantly; the surface upon which the canister sits can act as a heat sink (some a lot, some none); wind screens/reflectors can really play havoc with readings… and there are more.
I’d guess there is some standardized way to measure canister temperature — surely the DOT must have some sort of protocol because canister integrity at 50°C is their benchmark — but you’ll get all sorts of numbers depending on where you put the probe. With any canister, either full or partially full, you’ll get a different number if the probe is located above or below the liquid.
This is why IME and IMHO any reports of temperature must be taken with a huge grain of salt. The blizzard of variables is so overwhelming as to render almost any single number useless. The ‘touch test’ is as quick and practical as any method for ensuring a ‘safe’ operating temperature.
The only reasons I monitor temperatures are 1) to make sure the canister isn’t going to blow up in my face, 2) to get a rough idea yea/nay about the effectiveness, or lack thereof, for any modifications, and 3) to determine how close the canister is to the minimum temperature needed to vaporize the fuel in question.
The photo below is from one of the tests I did a few weeks ago when it was -5°F, with thermometer probes placed 90° around the canister circumference from the HX strip, and another one placed at 180° (opposite side of the can). Quite a difference in temperature only 90° and about 3 inches away despite the fact the can was in a cozy!
 Feb 27, 2016 at 5:29 pm #3385139
Feb 27, 2016 at 5:29 pm #3385139it seems like you want the sensor to be next to where the butane is, closer to the bottom
I wonder if the butane is at some particular temperature, will it be the same temperature on the other side of the steel canister? Steel isn’t a real good conductor. With your insulation outside the sensor it should make the sensor pretty close to the actual butane temperature though.
With your two sensors reading so far apart, are they both at the same level on the canister, both next to where the butane is? What if you put them both in a container of water, next to each other? That should be a good check. And if you put in half ice cubes and half water and stir a bunch, and place the two sensors in the center next to each other, you can see if they read close to 32 F.
Feb 27, 2016 at 7:09 pm #3385154“I wonder if the butane is at some particular temperature, will it be the same temperature on the other side of the steel canister?”
Yes – liquid butane temperatures will be pretty uniform. Both because of convection in the liquid butane, but also because if it is boiling on side (it’s boiling somewhere if the burner is on) and the liquid surface is cooler on the other side, vapor butane will condense on the cool side, bringing a lot of heat with it.
I do stranger and larger projects with propane and you can visually see similar amounts of condensation or icing all around the propane cylinder from the liquid level on down. The steel above the liquid will be warmer if the ambient temperature is warmer, so that no-condensation / condensation or no-ice / ice line is a clear indication of the liquid level. Even if there’s no condensation because of dry air or modest evaporation rates, I’ll run my hand down the cylinder and the transition from warm steel to cooler steel (adjacent to liquid) is easy to feel.
I agree you want the Moulder Strip© (™) to contact the canister as low as possible – so that it remains effective when the canister is almost empty. I’d put any temperature sensor low as well – so they read liquid temps throughout the canister’s life.
Bob’s post (immediately above) shows only a 9F difference between two spots on the canister. If the canister wasn’t full, height of the sensor will be a significant factor. Repeated with the same sensors in the opposite spots, the observed difference might have reversed if their height or contact area differed.
Feb 28, 2016 at 8:41 am #3385251I had a longer post all set to go, but I have done enough testing to satisfy (to myself, at least) the two main requirements: 1) it doesn’t overheat, even with fairly flagrant abuse, and 2) it’ll run a canister completely empty of n-butate at -5°F. The pharma folks would call that ‘safe and effective’.
Many years ago I read an article in Natural History magazine about Inuit and sled dogs and how they went about selecting and breeding them. The author sought out the oldest remaining Inuit he could find to ask him about the procedure, expecting some magical insight about subtle physical or behavioral characteristics known only to the natives after many generations of observations. The author was surprised and amused at the actual answer: “Well, we picked the ones that would pull the sleds and wouldn’t eat the kids.”
Sometimes that’s what it comes down to, and that’s enough.
Feb 28, 2016 at 9:10 am #3385264“The steel above the liquid will be warmer if the ambient temperature is warmer, so that no-condensation / condensation or no-ice / ice line is a clear indication of the liquid level.”
When I operate a canister near 32 F, particularly if it’s mostly empty, it freezes up on the outside showing where the liquid butane is
Feb 28, 2016 at 9:35 am #3385273If Bob’s sensors are positioned the same, the temperatures make sense to me. I’ve found that while I can’t possibly touch a hot copper strip, the canister is only “warm-not-hot” an inch away from the strip. The canister is cooler farther away from the strip of course. When not using a cozy, the canister is close to ambient temperature 180 degrees from the strip. This leads me to believe there is little danger of overheating a canister when employing this technique…
…even when using an insulating cozy. A couple of days ago I wanted to test a neoprene cozy that I’d made from a sleeve of a discarded wetsuit. I needed to know that the copper strip wouldn’t melt the neoprene. I placed a strip of carbon felt between the copper strip and the cozy to protect the neoprene, and I ran the stove for 10 minutes on a 40* F day. I constantly monitored the top of the canister with my finger, and it never got very warm at all on the side opposite the copper strip. 10 minutes was enough time to see if the neoprene had melted, and I was leery of running the stove much longer with the copper strip on such a warmish morning. Certainly at these temperatures a Moulder Strip wouldn’t be needed anyway. The neoprene was unaffected by the heat, I’m happy to say!
 Mar 8, 2016 at 10:24 am #3387566
Mar 8, 2016 at 10:24 am #3387566I was screwing around with this a bit.
I attached a silicon temp sensor to bottom of canister. Clamped it. Silicone to hold it and let dry. Remove clamp and put silicone where the clamp was:

Then I boiled 2 cups of water. Took temp data with Labjack module:

The stove burned for 2:40 starting at 0:00 on the plot. It took about 1 minute before the temperature propagated across the steel canister and sensor. The temp kept dropping for 2 minutes after I turned off the stove.
I estimated that the butane inside dropped 7 F from the initial temperature. There is a minimum temperature that the butane will boil at, which I guess is 22 F for the fuel in my canisters. As long as the outside temp is 7 F higher than this (29 F) this should work just fine.
I did this at about 35.5 F. This is the coldest it’s been in 2016.
I repeated for a 1/4 full canister:

I estimated the butane dropped 12 F. Assuming my 22 F minimum, then the coldest this should operate at is 34 F. You can see the temperature had increased to 36 F. It slowed down a little – took 3:00 to boil.
This is what happens as it gets close to the minimum, it slows down. As it gets close to the minimum, then it evaporates less, so it takes longer to boil.
A full canister would only drop about 4 F, so the minimum temperature I could operate at would be about 26 F. Plus the propane in the mix would help. I recently operated a full canister at 22 F and it slowed way down, but was still usable.
This is a strategy to operate an upright canister stove at cold temps – use a full canister.
Possibly a more expensive canister would have a lower minimum temperature.
Mar 8, 2016 at 10:35 am #3387571Another strategy is to warm the canister in your pocket or sleeping bag.
If the canister is full, it will drop 4 F for each 2 cups boiled. If you warmed it to 70 F, you could do 12 boils. Except it will also cool down from the outside air so it will be less, but that’s a lesser effect.
If the canister is 1/4 full, it will drop 12 F for each 2 cups boiled. If it started at 70 F, you could do 4 boils. Except it would be less than that from cooling from the air.
This was all with a 8 ounce canister. A 1/2 full 4 ounce canister would be the same as a 1/4 full 8 ounce canister. This contradicts some people’s experience, so…
This is theoretical so take it as you wish : ) I think it’s pretty clear this strategy works better for fuller canisters.
Mar 8, 2016 at 10:48 am #3387578Next, I made an aluminum “Moulder strip”.
Aluminum is half as conductive as copper so I made it twice as big as Bob’s copper strip. Heat loss is proportional to surface area, so I made 8 thin strips to make it about square cross section:

I used epoxy at the canister end to keep the 8 layers together. I put some conductive paste between the layers so they’d transmit heat better between them. The layer next to the canister is 1″ x 1″ for better conduction to canister. Grosgrain and ladderlock to press it against canister. I attached 2 silicon sensors to the strip about 1 inch apart:

It took a little longer to boil 2:55. The canister cooled down 10 F, a little less than the 12 F without the strip. So, you could say it would be good down to 22 F + 10 F = 32 F. Except you can see it takes a while for the heat to make it’s way down the strip and into the canister, so if it was colder, it would take longer than the nominal 3:00, but would be much better than without the strip.
It really needs to be colder here, like mid 20s to properly characterize this.
Mar 8, 2016 at 11:03 am #3387583I was on a trip recently when it was 22 F. I used a full canister. It was slow, but still usable. Consistent with my measurements.
I noticed the table I was on melted for about 6 inches around the stove. Must be radiant heat from the burner. If I could just direct this to the canister…

I took a 12 inch square of aluminum foil. 0.1 ounce. Placed it off center, and loosely folded the sides up to reflect the heat to the canister.

I stopped it at about 9 minutes but left the scale of the plot the same as the others. The canister started cooling after that.
This worked much better than the strip. The canister only dropped 3 F. Theoretically this should work down to 25 F. Except, like the strip, it takes a little while for the heat to make it’s way to the canister so it would probably work below this but be a little slower.
Again, if it gets cold, I’ll be able to characterize it better.
I think this is the easiest way to lower the operating temp of an upright canister. Even if the foil got holey or ripped it would still work.
Of course, putting canister in a container of water is also a good solution, but that’s a bit fiddly. As it freezes, put a bit of your heated water in. Heat of combustion is 100X heat of vaporization, so if you put 1/100th of your heated water into the container, that should be enough.
Mar 8, 2016 at 11:45 am #3387595Interesting stuff, Jerry. You have done some significant testing and recording. Tell me, what is the ratio of the fuels in the Burton canister? Is there much butane in the mixture, and what % is propane and also iso-butane?
I have been doing some desktop calculations of the theoretical performance of the MSR Iso-pro canisters (20% propane, 80% iso-butane, and supposedly zero n-butane) at various altitudes. We have good access to lots of altitude here in CO, of course. It appears that at 9400′ the propane vaporizes at -60* F and the iso-butane at -4* F, which should allow a canister stove with this brand of fuel to work. However, that hasn’t been my experience in the field, and I find that as things get below ~10* F at that elevation, the stoves perform rather poorly. Even on my 5440′ patio, where the propane should vaporize at -52* F and the iso-butane at +3* F, my stoves don’t do very well when the temperatures drop much below 15* F. There must be yet another variable that needs to be considered.
Mar 8, 2016 at 11:58 am #3387599How does the effect of altitude work inside the canister? To what extent does the liquid/vapor mix in the canister “see” the reduced atmospheric pressure? The steel of the canister could swell slightly, with the reduced outside pressure, and increase the inner volume of the canister, but this might be a pretty subtle effect.
As pressure of the atmosphere decreases at altitude, the back pressure through the regulator might diminish also, but is this a linear relationship? In other words, would the boiling point at of propane at 9400′ be the same in the canister as it is, uncontained? In an inverted canister, I think the fuel vaporizes after the regulator/valve, but in an upright canister, it has to vaporize in the canister, and leave the canister as a vapor.
Mar 8, 2016 at 12:44 pm #3387605That’s a good question, Jim. I know that Roger Caffin has debunked the concept of a regulator doing anything at all. He called the concept purely “marketing spin.” I do know that when I was testing various stoves a year ago, on my patio in minus 2 degrees F to plus 10 * F, the only stove I had that actually sort of functioned at +6* F without the aid of a hand warmer under the canister was my Jetboil Sol, which they claim has an innovative regulator in the stove. The canister had just 1.0 oz. of fuel remaining. In that test I had to open the valve all the way and it took a full 8 minutes to achieve a 2-cup boil, while consuming 7 grams of fuel to do so (vs. the usual 4.5-5.5 grams in 40-50* F weather). However, my Snow Peak Giga (which has no regulator) was able to perform OK at +10* F. It took 25% longer than usual to achieve a boil with a 1/2-full canister, but it used the same amount of fuel as usual (5.5 grams).
I really don’t know what this all means. Maybe a regulator will allow the stove to eek out a boil at temperatures below +10* F, whereas a non-regulated stove hits the wall at that point? Or maybe these were just random results from just one burn for each stove. If I’d done maybe 10 burns with each stove, I might have learned a whole lot more about regulated vs. non-regulated stove in muy frio conditions. But I stopped testing when I’d gone through 5-6 canisters, and after I learned the merit of heating up a canister using hand warmers. A couple of months later Bob Moulder introduced us to his “Moulder strip” concept, and my cold weather concerns are now history.
I still do wonder what the deal is with altitude though…
Mar 8, 2016 at 1:35 pm #3387620Max Burton canisters don’t specify the mix, but since it works at 22 F, must be mostly isobutane. They cost $4 for 8 ounces – you get what you pay for? Maybe what you pay less for is just the spec and actually they’re the same? Fred Meyers doesn’t sell them anymore, but now they sell Burton which works about the same, and the same price.
Actually, there’s a mix of chemicals that includes propane, isobutane, and nbutane. And the listed ratio is only approximate.
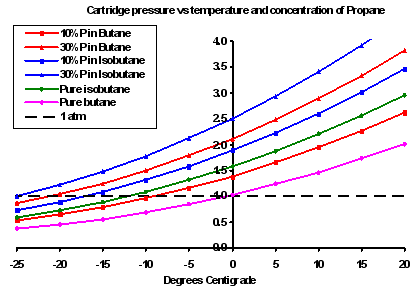
Operation at altitude – Roger has that plot of pressure vs temperature, e.g.

At sea level – 1 atmosphere – the dotted line, butane, is 0 degree C – so at sea level butane will work down to 0 C
At some altitude where the air pressure is 0.8 atmosphere, butane will work down to -5 C.
To generalize – at altitude, your canister stove will work at a little lower temp
Mar 8, 2016 at 1:42 pm #3387622I have Soto regulator stove. I like it.
It’s well made – Japanese engineering.
I like the regulator because especially when it’s cold, a minute after I turn it on, the temp will drop, which causes the pressure to drop. With a needle valve stove, the flame will turn way down. Very minor annoyance to realize the flame has gone way down so I have to turn needle valve up to compensate. This doesn’t happen with regulator.
It won’t operate at any lower of a temperature, as Roger wrote about.
Mar 8, 2016 at 2:02 pm #3387629“Even on my 5440′ patio, where the propane should vaporize at -52* F and the iso-butane at +3* F, my stoves don’t do very well when the temperatures drop much below 15* F.”
at 5440 ft you’re about 0.8 atmospheres. 20% propane/80% isobutane is about half way between 10% and 30% on Roger’s plot above, so you should be good down to -25 C = -13 F. But you say 15 F.
yeah, something weird here. Maybe I made calculation error?
15 F = -9 C. From Roger’s plot even (N) butane would work at 0.8 atmosphere.
Mar 9, 2016 at 8:22 am #3387758I like the regulator because especially when it’s cold, a minute after I turn it on, the temp will drop, which causes the pressure to drop. With a needle valve stove, the flame will turn way down. Very minor annoyance to realize the flame has gone way down so I have to turn needle valve up to compensate. This doesn’t happen with regulator.
As Roger has noted, and as I have observed and as you have mentioned, the regulator helps only in a very narrow temperature “window” for the canister. Above that narrow range you don’t need it, and below that range it doesn’t do anything.
The itsy-bitsy BRS runs like a blowtorch at -5°F without a regulator as long as the canister is warm enough.
Specifically concerning the regulated Soto, excerpted “bottom line” below:
<h3>My Conclusions</h3>
<h4>SOTO’s Constant Output Claim</h4>
The SOTO claim (from one of their documents) is that “The Micro Regulator Stove maintains a consistent output even when the fuel canister becomes chilled due to continuous use or cold weather.”Based on the data in all three graphs, this claim must be dismissed as totally false. The output at 18 C falls after a brief initial period as the temperature in the gas canister falls and the canister pressure drops. At lower temperatures, you don’t get any period of constant output at all. We may incidentally interpret this as meaning that the pressure drop required by the ‘regulator’ is so high that it is impractical for normal field use.
<h4>SOTO’s Output not Affected by Ambient Temperature Claim</h4>
One SOTO claim is that “The Micro Regulator Stove maintains a consistent output in hot or cold weather”. Other SOTO claims are “SOTO’s technology introduces the ‘Micro Regulator’ which boils water consistently and produces an even output regardless of the ambient temperature” and “Remarkable performance in sub-freezing temperatures.”Inspection of the three graphs shows that power output started at the same level at 18 C and 3 C (even though it rapidly declined thereafter), but at -1 C the power output was lower right from the start. And Randulph did not even get down to -5 C: his cold test was at -1 C.
The SOTO claim makes no mention of what will happen at -28 C, which strictly speaking is covered by this claim. It should be remembered that in Northern Europe (eg Norway), and in many parts of northern USA as well, walkers do sometimes experience that sort of low temperature. At that temperature, the canister will not be giving off any gas at sea level: the internal pressure is just too low. Not only will there be no pressure drop across the regulator, there won’t even be any pressure drop across the jet!
<h2>Summary</h2>
These results suggest that the entire advertising campaign for the regulator is based on vaporware. As you can see from the graphs, the SOTO OD-R1 pressure regulator offers nothing, except under hot weather for short periods. The claims are all marketing spin thoroughly contradicted by independent testing. Caveat emptor.On the other hand, the stove is very well made and works well. It would be a pity to discard it just because of the stupid marketing spin about the regulator.
Mar 9, 2016 at 8:32 am #3387759PS, I would also note that these “low temperatures” are laughably balmy compared to the sub-zero Farenheit temps at which we have already achieved stellar performance. Even the discussions about fuel mixes have largely been rendered moot — I’m running straight N-butane at -5°F, for goodness’ sake!

-
AuthorPosts
- You must be logged in to reply to this topic.
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Trail Days Online! 2025 is this week:
Thursday, February 27 through Saturday, March 1 - Registration is Free.
Our Community Posts are Moderated
Backpacking Light community posts are moderated and here to foster helpful and positive discussions about lightweight backpacking. Please be mindful of our values and boundaries and review our Community Guidelines prior to posting.
Get the Newsletter
Gear Research & Discovery Tools
- Browse our curated Gear Shop
- See the latest Gear Deals and Sales
- Our Recommendations
- Search for Gear on Sale with the Gear Finder
- Used Gear Swap
- Member Gear Reviews and BPL Gear Review Articles
- Browse by Gear Type or Brand.