Topic
Will an Aluminum Cook Pot work with a Wood Burning Stove?
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Home › Forums › Gear Forums › Gear (General) › Will an Aluminum Cook Pot work with a Wood Burning Stove?
- This topic is empty.
-
AuthorPosts
-
Mar 29, 2012 at 9:25 pm #1288065
After some internet searches trying to answer my own question I've decided to see what answers I can get on BPL.
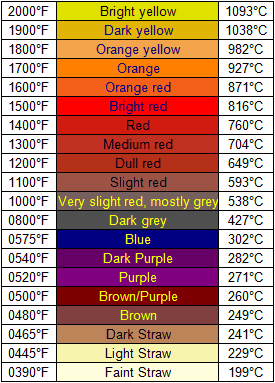
If the melting point of aluminum is 660.32 C, 1220.58 F and a wood fired stove produces an orange flame of 927 C, 1700 F will an aluminum cookpot such as an IMUSA 12cm mug survive repeated use over a wood fired stove?
I'm using this "Glow Chart" as some of my reference for the temperature of flames in a wood burning stove.

Ethanol burning in an alcohol stove burns at a lower temperature of 302 C, 575 F. I base this on the smokeless blue flame that it produces and the temperature reference on the 8th line from the bottom on the glow chart.
Does the cold water in the pot being brought to a boil or the food being cooked act as a heat sink to avoid a catastrophic failure of the cookpot / IMUSA mug?
Let my education begin. ;-)
Party On,
Newton
Mar 29, 2012 at 9:32 pm #1861328Works for Shug
 Mar 29, 2012 at 9:36 pm #1861331
Mar 29, 2012 at 9:36 pm #1861331Newton/John:
Yes, an aluminum pot will work — provided that you don't let it boil dry. If it boils dry, you're going to wind up with a pretty messed up pot. The water in the pot will boil at 212F/100C (adjust down if above sea level). The water is your "heat sink" and will keep the pot from getting too hot. Having said that would I use a really cheap, thin aluminum pot on a wood fire? Probably not. A good pot though should be fine.
Now, for a windscreen, aluminum can be problematic, particularly if you're going to try something like a Caldera Cone and you want to put the wood fire right up against the windscreen. That's not going to work very well in general (although perhaps there's some special alloy that could be used). For a windscreen, you'd be far better off with Ti. Quick, name all the wood stoves built out of aluminum. Uh…
Mar 29, 2012 at 9:38 pm #1861332There are some extra assumptions that you can throw into this analysis. You assume that cold water in the pot is in continuous contact with the pot inner surface, and therefore it acts like a heat sink to prevent aluminum failure. What can you say about the top of the pot that is exposed above the liquid surface? No heat sink there. Maybe or maybe not the flame will get to it.
Now, what happens if you are distracted while food is cooking in the pot? The food in contact with the pot overcooks and retracts a bit from the pot near the bottom. Now you have no heat sink there.
I would hate to see that nice gumbo fall into the fire.
–B.G.–
Mar 29, 2012 at 9:51 pm #1861351Mar 30, 2012 at 2:26 am #1861392I don't know where that 'glow chart' came from but the bottom half is highly suspect. The Draper Point (525 C) is the temperature at which substances start to visibly glow, below that temperature you will see nothing.
2nd, the blue flame from burning ethanol is not due to anything glowing (incandescance). The blue is due to specific molecular energy transitions and are called Swan Bands (named after a fellow Scot). The flame temperature from burning ethanol will be around 2000 C (http://www.sbf1.sbfisica.org.br/procs/2003/R_estendido/c54.pdf)
Mar 30, 2012 at 2:42 am #1861393Hey, John. This is only an average chart. MANY chemicals can change this color chart and it is never a safe assumption to cross it over between fuels. It looks like this applies only to wood. Copper, ionizes with a green glow for instance at almost any temp. The flames of a average alcohol stove are considerably hotter than 575F. If left in a blue flame, a paper clip or the like will glow quite orange/red. Which is the correct temperture? Sodium has a wierd yellow as it burns. Lots of other examples. Anyway, the chart only applies to wood and NOT to other materials. And it is not absolute…just rough. Types of wood will change the color. How dry the wood is will change the color.
Ti will likely not melt in most of these temps. You can distort it at 2000F. Any pot that will hold water is fairly safe to use. But it is possible to reach the kindling temperature of aluminum with a wood flame. You can set a piece of aluminum foil on a fire and it will be totally consumed in a good hot fire, not just melted…
I don't really think this is much to worry about. Water boils at 212F(or there'bout) and you can always plan on having water in it. The pot is fine. The Caldera Cone can burn and melt if used with wood. The screen is simply not up to the task. I made several different styles and they simply do not take the heat. Aluminum, while lighter, has a lot of drawbacks in that there is a fairly close melting point and kindling temperature where it will actually burn…get soft and crumbly.
Water does indeed act as a sink with the phase change consuming a lot of heat to produce low pressure steam. Soo, even the side walls are protected.
'Corse, you knew that….
Mar 30, 2012 at 3:36 am #1861398"Let my education begin".;-)
And my education continues or as Benny Hill used to say, "Learning all the time".
Stuart said, "The flame temperature from burning ethanol will be around 2000 C".
If the melting point of aluminum is 660.32 C then even my Budweiser aluminum bottle "cat can" stove using Everclear for fuel could easily give me an aluminum pot meltdown if for some reason I let it go "dry".
James said, "Water does indeed act as a sink with the phase change consuming a lot of heat to produce low pressure steam".
So, if the low pressure steam is cooler than the melting point of the aluminum pot it too acts as a heat sink and protects the upper portion of the side walls, correct?
I can accept all of this but it leads me to another question. I currently use an aluminum pot…

…and an aluminum bottle "cat can" style alcohol stove.

What protects my aluminum alcohol burning stove, or any other aluminum alcohol stove for that matter, from the 2000 C flames of the burning ethanol? Why doesn't my stove simply melt down underneath my cook pot and collapse?
Does anyone remember Mr. Wizard on TV? LOL
I await my continuing education. Can I get CEUs for this thread? ;-)
Party On,
Newton
Mar 30, 2012 at 4:55 am #1861410Given that a burning flame is 'hot' and everything around it is 'cold', what matters is the heat output of the flame, i.e. Watts (or BTUs) and the heat capacity of the thing being heated. An alcohol stove does not have a huge output, 500-1000W at a guess. A pot of water has a huge heat capacity and so heats up slowly. An empty pot has a small heat capacity and will heat up quickly, but the hotter it gets the more heat it radiates so the max temp it reaches is the one where the heat radiated is equal to the heat absorbed from the flame. Not a simple thing to calculate. However, put a small piece of aluminium foil in the flame and it will melt. In the stove itself, the evaporation of the ethanol 'absorbs' heat.
Mar 30, 2012 at 6:49 am #1861427"Does the cold water in the pot being brought to a boil or the food being cooked act as a heat sink to avoid a catastrophic failure of the cookpot / IMUSA mug?"
Yes.
If you're very careful, you can boil water in a waxed paper cup or a plastic water bottle. (Not that the water boiled in plastic would be safe to drink, except in a hydration emergency.)
My aluminum pots have deformed very slightly on the bottom from using them over a fire and wood stove.
Mar 30, 2012 at 7:02 am #1861432Stuart,
"In the stove itself, the evaporation of the ethanol 'absorbs' heat".
No wonder why "stovies" are so interested in alcohol stoves.
If I am understanding this correctly the boiling alcohol is absorbing heat like the evaporator in an A/C system and thus protecting my stove. At the same time the alcohol vapors are feeding the flames and producing the heat that boils the water. While all of this is going on the water acts as another heat sink to keep the pot undamaged.
The way this works is so "cool" 'er "hot". ;-)
Party On,
Newton
Mar 30, 2012 at 7:11 am #1861434Andy,
I can live with that.
At $6.00 per 12cm IMUSA mug I won't be sweating a slight deformation from using them over a fire and wood stove.
I'm looking at the IMUSA 12cm mug because it nests so well in the IKEA wood stove that I built a while back.
Thanks for sharing your experience.
Party On,
Newton
Mar 30, 2012 at 7:19 am #1861438"You can set a piece of aluminum foil on a fire and it will be totally consumed in a good hot fire, not just melted… "
Theoretically, but I've picked an awful lot of other people's aluminum foil out of fire rings. It's brittle, broken into little bits, and has ashes embedded in it – but it's definitely still there. I haven't experimented but my bet is you need something with chimney or forced air action to reliably burn Al foil.
Mar 30, 2012 at 7:22 am #1861441Hi Newton,
The chart you found looks like it applies to blacksmithing. Above 1000F (in a darkened space) hot material will glow red, then orange, then yellow, etc. Sometimes called "black-body radiation." Blue is actually hotter than yellow–it's beyond the range of your chart. These colors are reliable for heated materials (metal, incandescent lightbulb filaments, etc). As pointed out up-thread, the color of a flame also depends on the chemistry of the flame, but it's worth remembering that the blue flame of a welding torch is a lot hotter than the orange flame of a wood fire.The colors on the chart below 1000F are a spectrum of colored oxides that form on polished steel at relatively low temperatures. Blacksmiths use them as a visual guide for tempering tools, ie, removing some of the stress from a quenched tool to balance hardness and toughness.
Mar 30, 2012 at 7:29 am #1861446"Does anyone remember Mr. Wizard on TV?"
Don Herbert.
I believe that he was a contemporary of Roger Caffin.
–B.G.–
Mar 30, 2012 at 7:48 am #1861458@ David and Will,
Thanks for the information guys.
@ Bob,
Was Roger a comtemporary or a pre-cursor of Mr. Wizard? ;-)
The next piece of this puzzle is getting all of the pieces and parts together.
I need to weigh my IKEA wood stove and I have an email it to Tinny at Minibull Design inquiring as to the weight of his lid for the 12cm IMUSA Mug.
The 12cm IMUSA Mug weighs 3.4 ounces. All totaled up with shipping included I am looking at an investment of $27.00 for one mug and one lid. The mug is $6.00 + $4.00 shipping and the lid is $12.00 + $5.00 shipping. One third of my cost will be shipping.
Party On,
Newton
Mar 30, 2012 at 7:51 am #1861459"Does the cold water in the pot being brought to a boil or the food being cooked act as a heat sink to avoid a catastrophic failure of the cookpot / IMUSA mug?"
Short answer: YES.
I have boiled water in a fosters can set right into a camp fire.
You can even boil water in a PLASTIC WATER bottle over a wood fire, so aluminum will do fine. Just use common sense an don't throw it in dry. You are over thinking it. Even if you threw a plastic bottle into the bottom a raging bonfire, the top might melt, but as long as there is water in it, the bottom won't melt until it has all evaporated away.The "waterless" top part of the can won't melt unless it is in direct contact of the flames intensity. Flames licking up the side usually aren't enough to melt the exposed aluminum.
http://www.youtube.com/watch?v=NI-ItTGKHuE
http://www.trails.com/how_775_boil-water-plastic-bottle.htmlMar 30, 2012 at 8:01 am #1861464"Was Roger a comtemporary or a pre-cursor of Mr. Wizard?"
I think one of Roger's students was James Watt. He was one of the original stovies.
How many of us were transfixed as we watched Mister Wizard on television?
–B.G.–
Mar 30, 2012 at 8:13 am #1861472As plastic is an a good insulator, won't the outside of the plastic bottle melt/burn/give off toxic fumes?
Mar 30, 2012 at 8:20 am #1861478Yep, you can use a wood burner with an aluminum pot. I do it all the time with my hobo stove and my Heinie pot to save on fuel carried. As noted above, the water acts as an heat sink, raising the thermal inertia of the pot vessel as a whole, as well as becoming an heat exchanger as it boils. The energy needed to transfer the water from a liquid state to a gas state carries away an wholeheckofalot (technical term) of heat, which then rises with the steam and transfers to the atmosphere around it instead of staying in the pot and raising its temperature. The fuel in an alchy burner does the same.
All that being said, if you let your pot run dry…well…Bad Thingstm tend to happen. I've melted MSR Groundhogs (made of aluminum) down to slag trying to use them as pot supports with my hobo stove. These days, I've moved over to sixteen gauge steel wire (had it at home, so no extra investment) as a pot support, and it gets pretty hot–under the pot, it will turn fairly bright red. Thus far (about six months), no weakening of the supports has been evident.
Actually the finickiness (is that a word?) of the distance between a pot bottom an an alchy burner is mostly due a balance between actual heat on the bottom of the pot versus thermal feedback to the stove (which causes the alcohol to burn more quickly, lowering the efficiency past a certain point depending on the stove). Anyway, that's one of the reasons that playing with these little steel and aluminum contraptions is so fun.
Mar 30, 2012 at 11:19 am #1861570Um- I didn't read every word of every post, but I'm 99.9% sure that glow chart is used for estimating the temperature of STEEL. Take a clean piece of steel- lie one that's been freshly machined or sanded/ground clean. Hold it over a flame and it will start to change color as it oxidizes through the colors of that chart. Often time you sill spring steel refered to as blue tempered. That means that a piece of high carbon steel was heated to its austentine (?? been a few years, may be the wrong term) temperature (glowing red- molecules are free to be reorganized) and quenched. That is it's "full hard" state- i.e. brittle. Then it's tempered, and for a spring temper that means heating it until it turns blue, about 575F. Then when allowed to cool it's softened enough to bend like a spring w/o breaking, or so soft that it bends and stays bent. Again, this only works for steels. Aluminum, for example, doesn't change colors at all when it melts. This adds to the challenges of welding aluminum as there's no visual clue as to it's temperature. Ti changes colors, but I doubt it follows the same glow chart as steel.
As as for the temp of a flame- blue = hotter. The flame of an oxyacetylene torch is blue, but burns at about 5500F. A floppy orange flame is a lot cooler.
The flame of the alcohol stove is a lot hotter than 1000F. My stainless steel pot stand rods (3/32" 308ss welding filler rod) glows very orange, maybe even to the yellow orange range.

BM
(edit to add picture)
Mar 30, 2012 at 12:09 pm #1861598"As plastic is an a good insulator, won't the outside of the plastic bottle melt/burn/give off toxic fumes?"
I wasn't promoting the use of plastic pots on a regular basis, just using it as an example to support the fact that aluminum is more than capable of surviving a wood fire when boiling water.
Apr 2, 2012 at 3:30 am #1862436I received an answer from MiniBull design on the weight of their lid for the 12cm IMUSA mug. It tips the scale at 3/4oz.
So the total for the mug and lid adds up to 4.15 ounces.
Party On,
Newton
-
AuthorPosts
- You must be logged in to reply to this topic.
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Our Community Posts are Moderated
Backpacking Light community posts are moderated and here to foster helpful and positive discussions about lightweight backpacking. Please be mindful of our values and boundaries and review our Community Guidelines prior to posting.
Get the Newsletter
Gear Research & Discovery Tools
- Browse our curated Gear Shop
- See the latest Gear Deals and Sales
- Our Recommendations
- Search for Gear on Sale with the Gear Finder
- Used Gear Swap
- Member Gear Reviews and BPL Gear Review Articles
- Browse by Gear Type or Brand.