Topic
PROPANE Material Safety Data Sheet
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Home › Forums › Gear Forums › Make Your Own Gear › PROPANE Material Safety Data Sheet
- This topic has 26 replies, 6 voices, and was last updated 4 years, 5 months ago by
 Roger Caffin.
Roger Caffin.
-
AuthorPosts
-
Aug 31, 2020 at 7:25 am #3674007
Recent discussions regarding fuel for canister stoves caused me to do some research.
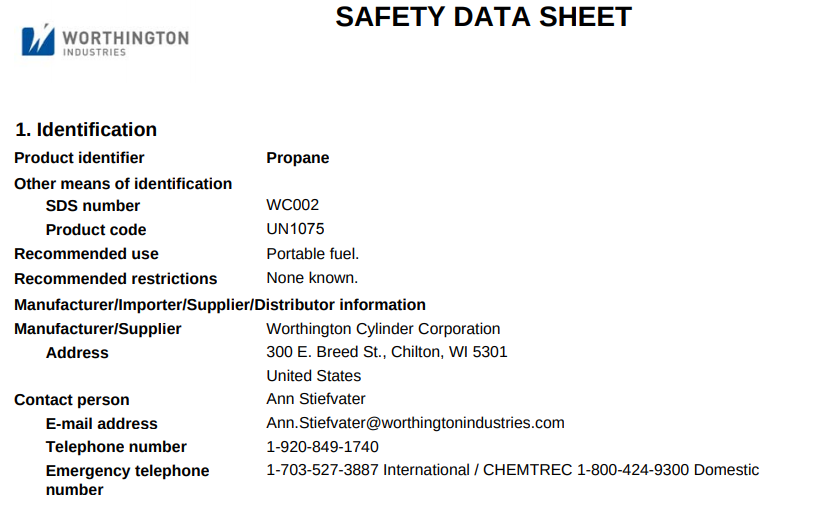
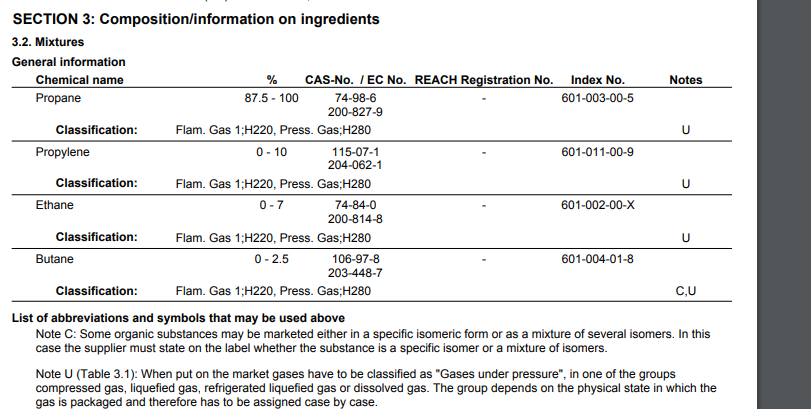
I wanted to know what the contents of a 1 pound canister of Propane was. This is what I found for a brand name “Worthington”:

 Aug 31, 2020 at 7:32 am #3674008
Aug 31, 2020 at 7:32 am #3674008 Aug 31, 2020 at 7:35 am #3674009Aug 31, 2020 at 7:48 am #3674011
Aug 31, 2020 at 7:35 am #3674009Aug 31, 2020 at 7:48 am #3674011And your point is …..?
Aug 31, 2020 at 9:02 am #3674017interesting
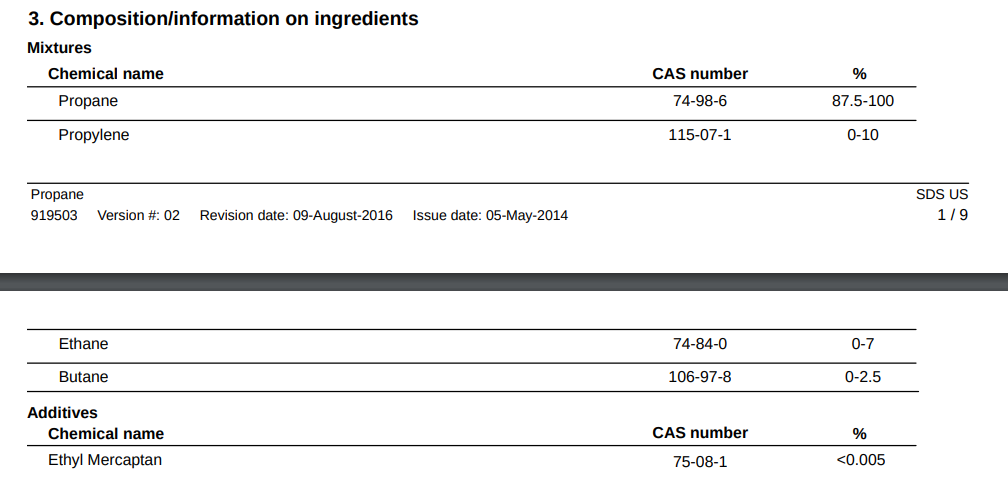
propylene and ethane have lower boiling points to propane, so it doesn’t matter much if they’re in the mix – -54 F, -128 F, -44 F
there’s only 0 – 2.5% butane which has a much higher boiling point, that’s close enough to zero as far as a low temperature camper is concerned. Maybe the last tiny bit of a canister
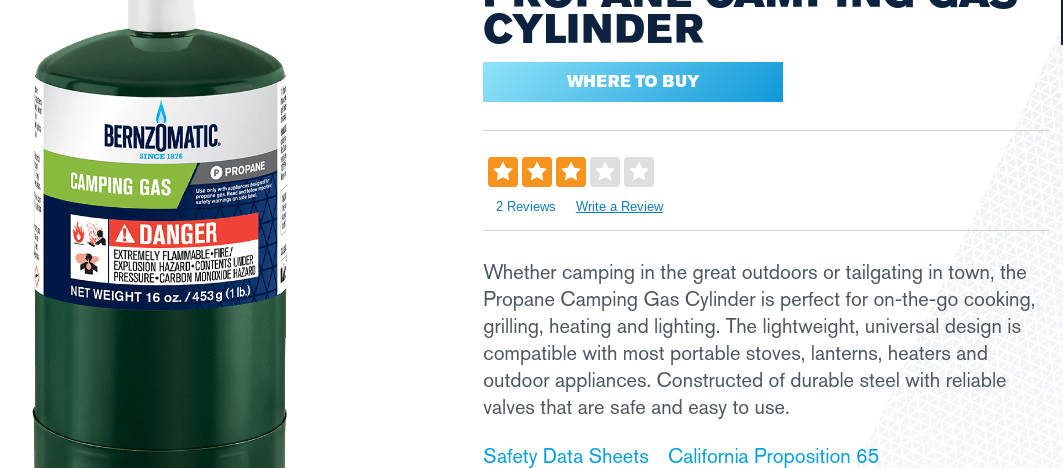
Aug 31, 2020 at 10:27 am #3674025Here is the safety data info for the Bernzomatic 16oz bottle of Propane camping fuel:
https://www.bernzomatic.com/Products/Fuel-Cylinders/Camping-Gas/TX916


 Aug 31, 2020 at 10:45 am #3674027
Aug 31, 2020 at 10:45 am #3674027That’s not as good as good butane like MSR Isopro
A little better than cheap butane
It would slow down when you get down to 32 F
No need for a heavy “propane” container
Aug 31, 2020 at 1:40 pm #3674052Yesterday in another thread we talked about refilling a small Boss canister.
If you read the above data sheets you might get one of those Ah-ha moments:-) and see the relation to what was said.
 Aug 31, 2020 at 3:50 pm #3674077
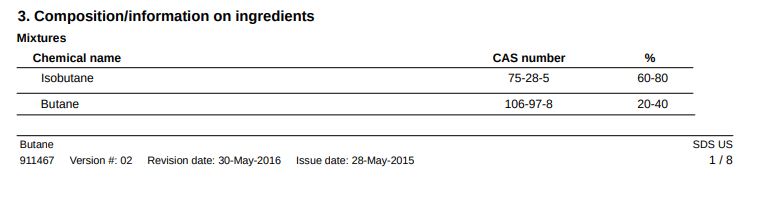
Aug 31, 2020 at 3:50 pm #3674077The SDS included in Dan’s 10:27 post is for a different product—a can of butane, not a propane cylinder. Where is this available? It’s probably cheaper than the good stuff at REI.
Aug 31, 2020 at 4:31 pm #3674087cheap butane is $1.25 per 8 ounces at the Korean grocer, approx 50% n butane, 50% isobutane. Good down to 32F or so.
plus one of these https://www.amazon.com/Adapter-Filling-Canister-Backpacking-Camping/dp/B08CBGXQQK/ref=sr_1_124?dchild=1&keywords=Butane+Tank+Connector+Cylinder+Coupler+Gas+Refill+Adapter&qid=1598913009&sr=8-124 $7.00
Aug 31, 2020 at 5:10 pm #3674094I really messed up on the post made at 10:27
The link should have been this:
 Aug 31, 2020 at 5:42 pm #3674100
Aug 31, 2020 at 5:42 pm #3674100good info at this link concerning propane/butane
https://www.quora.com/Is-there-a-difference-in-using-a-propane-torch-instead-of-a-butane-torch
Sep 1, 2020 at 4:22 am #3674160The MSDS is a legal document. It has to be accurate.
Yes, most propane has bits of other hydrocarbons in it. Distilling them out so the contents were really 100% propane (to lab grade) would make the commercial stuff far too expensive.
Cheers
Sep 1, 2020 at 6:31 am #3674169echoing Greg’s question, what are we learning here? I’m just curious and don’t really have a horse in this race. Can someone briefly decode what is going on here for muggles like me?
Sep 1, 2020 at 7:20 am #3674170:-)
In my original post I said:
Recent discussions regarding fuel for canister stoves caused me to do some research.
I wanted to know what the contents of a 1 pound canister of Propane was.
My ah-ha moment was finding out that the 1 pound tanks of Coleman Propane were not pure propane…..it’s a blend of propane and butane. Roger let me go on thinking it was 100% propane. So….I’m just sharing some information. You are right Matthew, you really don’t have a horse in this race. :-)
Sep 1, 2020 at 7:51 am #3674173100% Propane for Backpacking? YES!
https://adventuresinstoving.blogspot.com/2011/12/100-propane-for-backpacking-yes.html
quote:
What’s the “holy grail” of canister gas for cold weather backpacking? 100% propane. Propane is the best. Plain butane won’t vaporize below 31F/-0.5C. Even isobutane just sits there and looks at you below 11F/-12C. But propane? Propane vaporizes all the way down to -44F/-42C. Sweet!
What’s that you say? Propane is only available in those big heavy steel cylinders that are 16.4oz/465g net weight? And the total weight is even more than that? And you’re not about to carry that on your back?
Hey, I’m with you. Don’t blame you a bit. Those big green steel cylinders are just impractical for backpacking. Too bulky, too heavy, and the stoves that go with them aren’t any better. But what if there were a better way?
Sep 1, 2020 at 8:12 am #3674176“The Kovea LPG (Propane) Adapter”
https://adventuresinstoving.blogspot.com/2012/01/kovea-lpg-adapter.html
Quote:
I recently picked something up that turns out to be pretty useful: A Kovea LPG (Propane) Adapter. This adapter allows one to run a standard threaded canister gas type backpacking stove off of one of those green Coleman 16.4oz/465g cylinders of 100% propane, you know the typical propane canisters that everyone uses for camping stoves and camping lanterns for car camping.
I recently picked something up that turns out to be pretty useful: A Kovea LPG (Propane) Adapter. This adapter allows one to run a standard threaded canister gas type backpacking stove off of one of those green Coleman 16.4oz/465g cylinders of 100% propane, you know the typical propane canisters that everyone uses for camping stoves and camping lanterns for car camping.
When I tested the adapter, I didn’t need to use the set screw. In other words, it worked just fine out of the box, but it’s nice to know that it’s there if you need it. Now, a word of warning: propane is going to have a higher vapor pressure than the gasses typically used for backpacking. Yes, the gasses typically used for backpacking include propane, but not 100%. Usually propane is no more than about 1/3 of the total mix.
Higher vapor pressure could mean higher danger, so pay attention. If the pressure is too high, you could get flame “lift off” where the flame is blown away from the burner. In that situation, the flame will frequently die out while the gas is still flowing.
OK, let me get this straight. I’ve got a hot stove with the flame out but the gas is still gushing out. Um, couldn’t that be a little dangerous?
Why yes, as a matter of fact it could. Gas + air + heat = KABOOM! If you’re lucky, all you’ll lose is your eyebrows. If you’re unlucky, you’ll be finding out for 100% sure whether or not there really is a God, if you know what I mean. So, warning:
Propane is a highly flammable and potentially explosive gas.
Backpacking stoves are not designed to operate on 100% propane. Use at your own risk. Risk includes loss of property, serious bodily injury, and death.
The first rule, if you’ve made the decision to accept the risk of using a backpacking stove with 100% propane, is to turn things down low. Start low, and turn things up slowly. There’s no law that says you have to open the valve completely, so don’t.
Sep 1, 2020 at 8:27 am #3674178“Refilling Gas Canisters”
https://adventuresinstoving.blogspot.com/2012/01/gas-canister-refilling.html
Sep 1, 2020 at 8:40 am #3674182I thought the MSDS and everything else was pretty useful
The propane is not pure propane but it’s the same as
The other gases have an even lower boiling point except the butane, which is 0 – 2.5%, which is insignificant
Sep 1, 2020 at 9:00 am #3674185What’s not clear from that text you posted, is that the risk of putting propane in a canister is that it will burst if it gets too warm
Normal canisters are rated for 110 or 120 F. With propane it will be less. It also depends on how much margin they added. Roger’s article about exploding canisters has a number for the actual temperature for that canister.
One problem is transporting the canister. If it’s in a hot car it could get up close to that 110 or 120 F.
The other is operation – it can heat up from the stove. Make sure and feel the side of the canister, where the liquid is. It should be less than 110 or 120 F for normal canister. Some temperature less than that for propane.
I think there are too many unknowns to calculate what temperature you can safely operate at, what proportions are in any canister or cylinder,…
One way to get a good idea what’s in a canister is to put in a freezer and find the boiling point – the temperature where there is just barely any pressure, you can just barely smell gas coming out if you open the valve.
Like I said, try it, put it in a place outdoors where it’s warmer than you’ll ever experience and make sure it doesn’t burst.
If it’s warmer than about 35 F, you don’t need propane, better to just use butane.
Sep 1, 2020 at 4:19 pm #3674304Jerry – if you really want to know what is in the ‘propane’ you would probably need a mass spectrometer. Slight variations in pressure and boiling point would not be enough, down at the 1-2% level. They are expensive things.
———My ah-ha moment was finding out that the 1 pound tanks of Coleman Propane were not pure propane…..it’s a blend of propane and butane. Roger let me go on thinking it was 100% propane.
Sigh.
Wrong.Industrial chemicals (and propane IS a chemical) are never really pure. In general they don’t need to be 100.00% pure, and the energy cost of getting rid of all the ‘contaminants’ is just not worth while for commercial use. The propane you get from an LPG bottle is ‘mostly’ propane, but with other volatiles deriving probably from the gas well the propane came out of.
But I NEVER said that commercial propane was a deliberate blend of butane and propane. I never said that because it simply is not true. I may have used the phrase ‘100% propane’ as a descriptor for what you get in something like a green Coleman propane bottle, but only to distinguish it from a 30%/70% blend.
Come to think of it, I wonder exactly what is in the cheap flyspray cans of ‘butane’? Testing reported here at BPL makes me think it is a rather crude mix of iso-butane and n-butane, probably with some propane and other volatiles, but other than that guess, who knows?
What is going on here? I don’t know, except that I do get a bit tired of being constantly misrepresented and misquoted. It gets boring.
Cheers
Sep 1, 2020 at 5:59 pm #3674338I don’t need to know accurately what’s in there
Finding the (freezing) temperature where the pressure drops to zero is really all I care about. An upright stove will operate pretty good 10 F warmer than that. An inverted stove would operate just a few degrees F warmer than that.
Second order – would be to use up most of a canister and repeat. If a canister had a mixture of propane and butane you’d figure that out, that is, you’d figure out what temperature it would operate at.
Sep 1, 2020 at 6:00 pm #3674339I don’t think it matters what somebody though somebody else said, or anything else like that
What’s important is just figuring out how to operate a stove at how low a temperature
Sep 1, 2020 at 7:48 pm #3674369Thanks Dan :)
Sep 1, 2020 at 7:49 pm #3674371What is going on here? I don’t know, except that I do get a bit tired of being constantly misrepresented and misquoted. It gets boring.
Cheers
Roger, see my last post on page 3 of the following thread:
-
AuthorPosts
- You must be logged in to reply to this topic.
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Our Community Posts are Moderated
Backpacking Light community posts are moderated and here to foster helpful and positive discussions about lightweight backpacking. Please be mindful of our values and boundaries and review our Community Guidelines prior to posting.
Get the Newsletter
Gear Research & Discovery Tools
- Browse our curated Gear Shop
- See the latest Gear Deals and Sales
- Our Recommendations
- Search for Gear on Sale with the Gear Finder
- Used Gear Swap
- Member Gear Reviews and BPL Gear Review Articles
- Browse by Gear Type or Brand.