Topic
Primus Winter Gas Review
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Home › Forums › Campfire › Editor’s Roundtable › Primus Winter Gas Review
- This topic has 47 replies, 19 voices, and was last updated 8 years, 9 months ago by
 Roger Caffin.
Roger Caffin.
-
AuthorPosts
-
Dec 23, 2015 at 4:54 pm #3372100
Has anyone tried these butane/lindal adaptors from taiwancamping.net? They are much cheaper pricewise but not sure about the quality.
http://store.taiwancamping.net/home/outdoor-gears
(scroll down a little)
Dec 23, 2015 at 10:46 pm #3372144Wow. Thanks much. I just spent an hour looking at YouTube videos on how to refill your canisters, mostly with butane, but there were even a few using a proportion (20-30%) of propane. The only one containing propane that sounded reasonably safe was the guy who made a mixture of propane, butane and isobutane directly into a propane tank (like for an outdoor grill) and then used that mixture to refill his canisters as needed. That wouldn’t help me since I often travel by air to these hikes and can’t mail refilled canisters. I’d imagine that even after shaking his large propane canister with each use, the propane will be depleted sooner than the other gases.
Does anyone know if cans of butane are usually available at roadside convenience stores in the US?
Dec 24, 2015 at 5:27 am #3372163Here in the ADK’s, no. Unless you stop at one of the major towns, refills are not generally available. Sometimes you can find the refill’s for a lightter, but these are invariably as expensive or more expensive than buying a regular can of fuel.
Dec 24, 2015 at 7:21 am #3372176$2.30 for a 230 g canister of iso-butane/propane – not bad – $5 or $6 here
he complained that was expensive so used his refiller ($8) to use $1 for 230 g of n-butane
the Burton brand iso-butane at Fred Meyers (for you PNW people) is $4. I’ve used maybe 10 of them without problem. They don’t say iso-butane, but I know they are, because they operate good below 32 F which n-butane would not.
Dec 24, 2015 at 12:37 pm #3372247I think this works because of the additional surface area of the paper. Here’s a thought experiment:
It’s often handy, when trying to understand physical phenomenon, to take the situation to an extreme and see how it behaves. For the extreme example here, make the canister 5-mm in diameter, but the same volume as a standard canister. That’s right, it will be several meters tall. Now fire up the stove, this can be done in warm weather. The stove will likely have trouble running, because all of the heat of vaporization must come through a very small surface area around the gas/liquid interface. There’s just not enough surface area to conduct enough heat into the fuel to evaporate it fast enough to maintain a large cooking flame.
The winter fuel canister has liquid fuel all around the canister, bottom, sidewalls, top, (the paper keeps it on the sidewalls and top) so it has maximized the surface area to conduct the heat required for vaporization of the fuel.
Another thing they could do is put the paper in an even larger canister. This should improve performance even more, because it increases surface area for heat conduction.
(I’m a mechanical engineer)
Dec 24, 2015 at 2:04 pm #3372263Maybe I’m missing something here, but…
Primus is selling the same weight of fuel in canisters that appear to have the same physical dimensions. The winter canisters have additional material inside, meaning that there is less volume available for the same amount of gas, thereby effectively increasing the pressure.
Did I get this wrong? If not, wouldn’t that have an effect on stove performance?
Dec 24, 2015 at 2:34 pm #3372268The stove will likely have trouble running, because all of the heat of vaporization must come through a very small surface area around the gas/liquid interface. There’s just not enough surface area to conduct enough heat into the fuel to evaporate it fast enough to maintain a large cooking flame.
I’m sorry, but this description of how the energy might get into the fuel is totally wrong. The energy does NOT come in through the tiny liquid/gas interface. It would come in from the side walls, which in this case would have a very large surface area.
The rest of the posting (‘so it has maximized the surface area to conduct the heat required’) is also entirely wrong too.
Cheers
Dec 24, 2015 at 2:38 pm #3372271meaning that there is less volume available for the same amount of gas, thereby effectively increasing the pressure.
Sorry, but you did get that completely wrong. The pressure is set by the temperature and the chemicals involved. Vapour will be created to make up that pressure.
Otherwise, yes, there is little difference between the canisters.
Cheers
Jan 1, 2016 at 1:21 pm #3373521Hi Maxine
It’s a common topic. The threads Bob has pointed to are good.
Short answer: yes you can but it is a bit tricky to do, and it should be done OUTSIDE! But plain butane – only for warm weather.
Cheers
Jan 1, 2016 at 2:59 pm #3373531I think I didn’t make myself clear.
Thought experiment:I have 100g of some fuel mixture, and place 50g of that mixture in each of two containers– 1 container at 100cc volume and the other container at 50cc volume. The pressure at the start would be the same in both containers, but I think the smaller container would be able to produce/maintain that pressure for a longer period of time, since it would require much less vapor to do so. This would essentially improve performance at the end of the cartridge life.
Where did I go wrong this time?
Jan 1, 2016 at 3:05 pm #3373532“Primus is selling the same weight of fuel in canisters that appear to have the same physical dimensions. The winter canisters have additional material inside, meaning that there is less volume available for the same amount of gas, thereby effectively increasing the pressure.”
Not entirely wrong but everyone assumes that temp would remain a constant. If all is at a steady state, then there is no effect. Sort of like the oxymoron of holding your pee will keep you warmer because when you take a leak it steams in cold weather therefore it must be releasing heat. No. Nothing happens except a slight decrease in overall mass. If it is at the same temperature, nothing happens, thermodynamically speaking. If you increase pressure, then some gas will be forced back into a liquid phase in a closed container. When this happens there is an increase in temp in the whole container. Though Roger assumes it only happens at the surface where the phase change actually occurs. It actually happens throughout the fuel since the tendency to evaporate or remain a liquid depends on the random motion of the molecules throughout the canister. Temperature is the velocity of the molecules. Space, as an example, can be extremely hot because the molecules usually move very fast. But, because there are few of them, they do not offset conduction or radiation so it “feels” very cold. Canisters absorb heat and radiate heat simultaneously. It is the difference between the two that we usually look at. It is the same for evaporation and condensation. Even though they happen pretty much simultaneously (what we call vapour presure.) So, pressure can also distribute the heat throughout the canister. But in a steady state system, adding a blotter paper will have no effect other than to increase the volume of fuel available to output and maybe increase the insulation (good or bad.)
Jan 1, 2016 at 7:56 pm #3373558Hi James
When this happens there is an increase in temp in the whole container. Though Roger assumes it only happens at the surface where the phase change actually occurs. It actually happens throughout the fuel
I think we are talking about different time scales here. When a molecule of vapour becomes attached to a liquid surface, the energy of the vapour molecule is absorbed locally – at the surface, at that instant. But of course in a very short time Brownian motion in the liquid will redistribute that energy around the bulk of the liquid.
Cheers
Jan 2, 2016 at 7:10 am #3373587Yes, just so. I would be interesting to see a chart of *rates* of propagation of pressure in a liquid/solid. I am not sure they even have these charts. Most everyone just treats these as instantaneous.
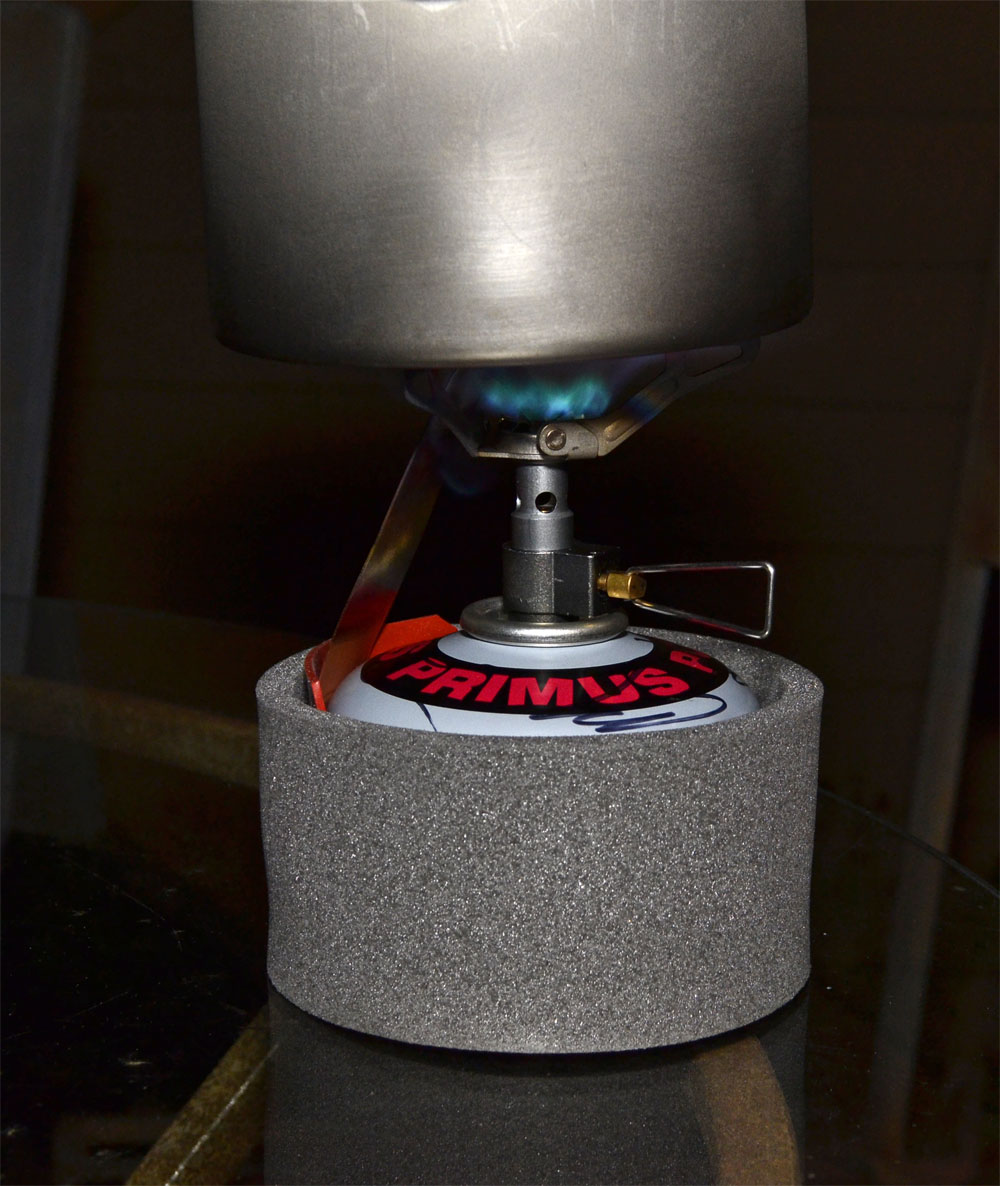
Jan 4, 2016 at 6:13 pm #3374004Result of first tests with pure n-butane: simply WOW!
Lucky me. A real winter cold front finally moved in, and the cheap refill adapter mentioned above arrived in the mail today from Taiwan. Pretty crude compared to the other, much better adapter that is enroute from Japan, but it worked well enough. I was able to transfer 91.5g of n-butane to an empty Primus 100g canister, and it was quite easy. There’s no clamping or linking mechanism, which meant I just had to hold the butane can and the Primus canister together for about 3-4 minutes, although that process was interrupted by a weight check on the digital scale to make sure I wasn’t overfilling. But it works.
First test protocol: Ambient temperature was 13°F (-10.5°C). Stove was BRS3000-T. The canister was room temperature of 72°F, with HX strip in place and in the cozy. Took it out on the deck and let it run with an MSR Titan kettle and some water. Never missed a beat and boiled the water, but it took considerably longer than normal because it was windy and I made no attempt to block it.
***No effort was made to time the boil nor to measure fuel efficiency. I call this sort of very preliminary test a “Gross Effect Test” that is ntended only to discover if it would work at all. After all, if you can’t get past this point there’s no use proceeding anyway.***
Second test protocol: Canister out of the cozy for 45 minutes to let it cool down to ambient, same as above at 13°F. Put the canister (with HX strip) back into the cozy. Attempt to light it and, of course, it won’t start. Placed my bare thumbs on top of the canister for about 30 seconds to warm it up a little, then take another shot at lighting it. It lights, but the flame is extremely weak. However, I just want to see if it can get the feedback loop going by itself, and it does. It takes 3-4 minutes, but it is running at full power. I also notice that the feedback loop picked up speed when the MSR kettle was placed on the stove, with the base of the kettle deflecting the flames more directly at the HX strip. Had I realized this earlier, I am absolutely sure that the feedback loop would have been established more quickly.
Bottom Line: With the HX strip I can now use plain, n-butane in very cold weather. I would never have thought this possible. This could prompt a very serious re-evaluation about the need for propane and isobutane.
Some photos…
Ambient temperature of 13°F (-10.5C)

HX strip setup during test

Achieving a boil easily at 13°F with pure n-butane
 Jan 5, 2016 at 1:57 am #3374049
Jan 5, 2016 at 1:57 am #3374049Hi James
see a chart of *rates* of propagation of pressure in a liquid/solid.
Basically that’s the speed of sound in the liquid. Search on ‘speed of sound in butane/propane/LPG’ and you will get a value a bit over 800 metres/second.
Rate of diffusion in the liquid would be different, and harder to measure.
Cheers
Jan 5, 2016 at 2:00 am #3374050Hi Bob
Don’t forget: a canister carried in your pack will probably be above 0 C, even in very cold weather. That helps.
I normally store the canister overnight at the foot of my quilt. That helps too.
Cheers
Jan 5, 2016 at 7:22 am #3374059Yup, I had forgotten about that. Thanks!
Jan 17, 2016 at 5:37 am #3376270I don’t understand Roger. He claims to be scientific researcher and as such he ought to know that no matter how beatiful a theory is, if nature does not verify the theory, the theory is worth nothing.
I don’t see Roger objective in his studies and writing. He has for many years been negative towards Primus of some reason I still not understand. He clearly critises Primus in his email conversation even before he made a field test!
I don’t care that Roger got the wrong delivery. It sounds like he first blame Primus for it and later the shipping company. What does it have to do with the test?
Roger writes the test result as support Primus that the winter canister works. THerafter there is explanations that is not supported but any test result. Rogers suggestion that it the infrared light from the stove as is the explanation for way the winter canister works. My observation when I use external stove is that it is the lower part where the liquid is that is getting frosty not the whole canister. And that no matter if the canister is upside down or not. Roger would easily proof his case by measuring the temperature of the canister with a laser-thermometer. And all backpackers can then happily by the cheapest canister and paint it with suitable paint.
An experiment about evapouration, everybody knows clothes dries faster if put them on a line that letting the lie in a pile. What happens when you put the clothes on the line is that you increase the surface area!
Another experiment. Dip a finger in water and thereafter put it up in the air (outside, in the house). The side of the finger as is hit by the wind will colder! The general explanation is that molecules need extra energy to be able leave the surface of the finger and it gets that energy from the finger as then gets colder.
These two experiment indicate that winter canister ought to work. Hardly any effect when the canister is full since most of the paper is below the surface. More effect when the canister is close to empty. Since the liquid and paper gets cold when the gas evapourates the effect will be less if the stove is burning a longer time compared to just boiling up water during short time. Of the article it is not possible to see if Roger or Primus has made such comparison.
My observation on the frost on the canisters, when they are in use, is that frost is up to the level of the liquid inside. This clearly visible when the canister is half full. No matter if the stove is on top of the canister or on the side.
As Roger showed in the Ideal gas law, an increase of temperature of the gas will increase the pressure. So cold days I chose to make sure gas gas is warm. I solve that in two ways. One way is to have the canister inside the jacket when I go out. At time of cooking I put the canister in a fleece bag especially made for that canister size, so the gas is warmer than the surround air. The other way I make sure it is warm is by using a hand warmer, you know the ones as you boil in water and has a small metal button as you click when you want a chemical reaction to release heat. Since it is very cold outside there is no risk that the canister will overheat.
In the end Roger comes to a valid conclusion. The winter canister works, but it is up to the user to decide if it is worth the higher price.
I will find it interesting if Rover makes a test, testing if painting a canister will have an effect. It will be interesting to read no matter if it has an useful effect or not. Such a test could revolutionary the market if he is correct, and I will be the first congratulate him for his valuable research.
Jan 17, 2016 at 1:26 pm #3376333Hi Harry
I agree totally with you about the difference betwen theory and experiment.
I mentioned the shipping problems for two reasons.The first is because I found the problems amusing in some ways, and I thought that others might also be amused. Light entertainment.
The second reason is more important. By the time the canisters finally arrived, it was late spring heading into summer – in Australia. Your commnt “Since it is very cold outside ” is therefore slightly off: it was over 30 C. I could wait 6 months to do further testing, or I could do the best the weather conditions permitted and publish quickly.
For the record, I don’t think that painting the canisters would do very much: a degree or two at the best. Would it be worth doing? The alternative is to use a readily available remote inverted canister winter stove.
Cheers
May 24, 2016 at 11:17 pm #3404593Great review! I agree with inverted for cold weather performance with the caveat of needing a filter just before the jet and a field service kit. If you use upright in the cold, warm the canister up in sleeping bag or jacket and put in a neoprene cosy on it. I have various sizes that I buy to protect camera lenses. The have draw cords and double as a GPS protector.
May 24, 2016 at 11:29 pm #3404596the caveat of needing a filter just before the jet
It turns out that having a filter before the jet can be really really bad with some canisters. The filter can block up with very fine dust and higher paraffins fo8nd in some – especially Chinese ones, very quickly, whereupon the stove just dies. This will be covered in an article to be published soon.
Cheers
May 25, 2016 at 12:07 am #3404600Thanks for the warning with filter clogging Rodger. I did also add “and a field service kit” I have made up my own kit with spare filter material, jet tip cleaner to match cooker, jet removal tool, 2 x canister O rings & a flint lighter as my cookers have piezzo igniters. I’ll be very interested to see the article to be published soon. In the mean time I can forgo the filter and just clean the jet as required. Is the filter cause a safety issue or just an issue that is more difficult to fix if you don’t have a reamer/tip cleaner?
May 25, 2016 at 12:30 am #3404602Hi Ebbo
I was field testing a new stove, which will be revealed soon, up in our Alps. I was trying to cook dinner, and the stove kept dying. Eventually I removed the jet and examined the filter under it. It was wet with oily stuff, and there was a dark patch in the middle. I thunked a bit, removed the filter, and cooked dinner.
Later on at home I decided to do some more checking on this. I found that a second filter which was in the canister connector was also blocking the flow, again with oily stuff (just below a wax i think) and very fine dust (microscope this time). The Chinese do not filter their gas the way western companies did – filtering costs money.
Service kit? Oh YEAH, absolutely. See our Stove Maintenance article on this.
Cleaning jets: I will recommend fine hard copper wire as this is softer than the brass used to make jets. You do NOT want to damage the jet hole at all. If you have an old-fashioned ‘stove pricker’ which is small enough, that’s fine, but the word ‘reamer’ would really worry me. OK, I am fussing.
Cheers
-
AuthorPosts
- You must be logged in to reply to this topic.
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Trail Days Online! 2025 is this week:
Thursday, February 27 through Saturday, March 1 - Registration is Free.
Our Community Posts are Moderated
Backpacking Light community posts are moderated and here to foster helpful and positive discussions about lightweight backpacking. Please be mindful of our values and boundaries and review our Community Guidelines prior to posting.
Get the Newsletter
Gear Research & Discovery Tools
- Browse our curated Gear Shop
- See the latest Gear Deals and Sales
- Our Recommendations
- Search for Gear on Sale with the Gear Finder
- Used Gear Swap
- Member Gear Reviews and BPL Gear Review Articles
- Browse by Gear Type or Brand.