Topic
Selecting a Canister Stove for Cold Weather BackpackingPart I: Stove and Fuel Fundamentals
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Home › Forums › Gear Forums › Gear (General) › Selecting a Canister Stove for Cold Weather BackpackingPart I: Stove and Fuel Fundamentals
- This topic is empty.
-
AuthorPosts
-
Mar 28, 2006 at 1:43 am #1353602
> Here is my theory on how the safety shut off works. Refer to the last picture I posted. –
The dowel in the center of the red plunger is in a hollow recess. This allows the hot butane vapors to swirl around the dowel softening it until it mushrooms under pressure from the spring while the rest of the plunger remains functional and cooler due to its greater relative mass. Once mushroomed the plunger reseats itself against the neoprene gasket cutting the flow of gas. The canister becomes unusable even though it may contain gas.Don’t get too excited about the shape of the red plastic valve. Different brands of canisters, and slightly different models, have different shapes for that bit. Lindal can make a lot of shapes …
As you note, it’s an open/shut valve. Fine control is done by the needle valve in the stove.
If things go mushroom shaped, the internal gas pressure forces the softening red plastic up against the neoprene to seal the whole thing off. Damn good thing too. :-)Mar 28, 2006 at 7:10 am #1353617Quick update: just tore apart a valve mechanism, and should be able to accomplish the thermal testing on the “blue” and “red” plastic today or tomorrow. This should give us a good indication of the temperatures they withstand before softening. Will keep you posted. (for the technically minded, the technique we are using is TMA – Thermal Mechanical Analysis, and we are looking to determine Tg, “glass transition temperature”.
Roger – I hear your request for graphs…will try to comply!
Mar 28, 2006 at 8:35 am #1353622I bought a canister and showed it to my friend the Engineer. When he saw the concave bottom, his first words were “aah, there is the safety mechanism.”
The “pop-out” bottom is a safety mechanism: the canister is much weaker in its’ standard shape as there is multi-directional stress on the concave bit. When it reaches “pop” temperature, it expands to be egg-shaped (convex bottom) and in doing so:
a) signals you that you are a Darwin Awards candidate and you have made the short list
b) increases internal volume significantly, thereby decreasing the pressure
c) becomes much stronger. In “egg” shape, there is a uni-directional force exerted relatively evenly on all surfacesNot that anyone here should even think about relying on the canister pop (or any “self-sealing” behavior) as a failsafe.
Interestingly, the Coleman (30% pro/70% bu) canister states 50 degrees C as a maximum storage temperature. 50 is fairly hot to the touch IMO, and then you’d have to figure in an “engineering factor” to get to a true “pop” or engineered failure point for the canister.
Which just makes me want to know even more!
Mar 28, 2006 at 12:13 pm #1353643The data are in! (and thank you to an interested and generous co-worker). Looks like the blue and red pieces are both the same material. Unfortunately, it looks like there is not a really, really, clear-cut answer for us: this material exhibits a very, very broad softening range….starting at about 50C, and ending (rather catastrophically) at about 175C. Between those temperatures, it progressively softens. A raw crunching of the numbers puts would indicate that we would really start being concerned at a temperature above about 100C (for the techies…this would be the indicated Tg measured from loss-modulus).
I think the bottom line would be as follows: below 50C is just fine. Between 50C and 100C there is some risk to warping the red pin and shutting down the gas flow. Above 100C you are running on borrowed time, and the “shutoff mechanism” just mentioned by Roger (i.e. “mushrooming” of the pin) is much more likely to occur.
Roger – I know you want plots: I am trying to paste the appropriate plots into this message…but can not figure out how to do it (the paste option does not work). Can someone enlighten me please?
Mar 28, 2006 at 2:18 pm #1353656Cushing,
Most people here use the free Photobucket service as an image hosting site. If you want, email them to me and I will do it for you.
Robert
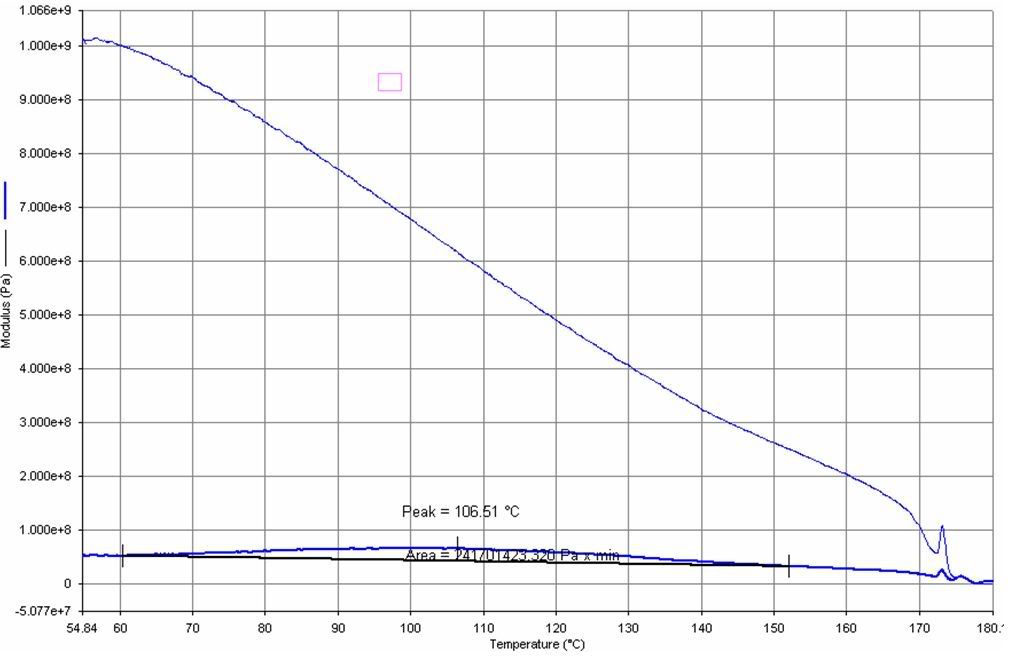
Mar 29, 2006 at 10:23 am #1353757Ok…lets try this. Here is an image of the data from the thermal analysis on the red piece from the stove. The top line is the elastic modulus (read “stiffness”) versus temperature (in degrees C). You can see that the modulus starts decreasing at 50C, and does so continuously until about 170C, at which point the part just plain fails. For our purposes, what we REALLY want to look at is the bottom line (the one with the “hump” in it. This is what is called the “loss modulus”…and is more of an indication of when the part really starts “flowing”..i.e. irreversibly deforming. This curve is very broad, with a peak at 106C (most materials have a much more sharply defined peak). Usually we would define the peak as the so-called “glass transition point”…but with this material with such a broad peak it is thus hard to define a specific temperature at which the material starts to “flow”. But we CAN say the following: below 50C (122F) the material will not flow. Between 50C and perhaps 80-90C we are probably ok…but there is some risk of the stove throttling down the gas flow. Above 106C you are REALLY running a risk of the valve shutting off…and forget it if you reach a temp above 170C.
Sorry the results did not turn out clearer…but at least this result is consistent with the observation that “not-too-hot-to-touch” is “ok”. Here is the link to the plot. (did I put this in correctly Robert…or can I improve on the way the image is posted? How did you actually get your pictures imbedded into your posting?)
Thank you Douglas for giving the info on displaying the image….Figured out how to get the image larger….hopefully not too large now.
 Mar 29, 2006 at 12:22 pm #1353765
Mar 29, 2006 at 12:22 pm #1353765>How did you actually get your pictures imbedded into your posting?
You provided the URL to the picture in your post. All you need to do is insert:
<img src=”
before the URL and add:
“>
after it. That tells the web browser to display the image in the post. Try it! Just EDIT your post and make the change.
The link in the previous message to Photobucket is done as follows:
<a href=”(URL goes here)”>(text to click on)</a>
E.g., <a href=”http://www.photobucket.com/”>Photobucket</a> will show up in the post as Photobucket.
(Edit: Cool! You got it!)
For bigger pictures, you probably have to go to a different service (sorry, I don’t have a recommendation) or if you have a free web site with your high-speed Internet provider, you can just make a directory there, add the pictures, and then reference them in your post (this is what I do).
Mar 29, 2006 at 3:16 pm #1353777This might have been commented on before – but one thing that could be done is to use temperature sensitive stick-ons. See URL below….
Mar 29, 2006 at 7:40 pm #1353795Thanks Cushing for the data and your analysis. Needless to say it is far better than I could have produced by pushing dull instruments
into plastic hot from my Magic Chef oven!I’ve never used Tempilabel but have used Templaq in the past. Earlier in this thread I googled it and discovered it had been used on
the X-2 rocket plane to obtain data on aerodynamic heating. It will probably suffice for butane canisters too.Thanks again,
Robertps – Cushing got a larger version of his chart up late Thursday afternoon. Hitting the refresh button on your browser will bring it up. If the refresh doesn’t work you may have to clear your cache.
Mar 31, 2006 at 10:57 am #1353901While I was looking into the canister fitting for the burst test, it ocurred to me that the metal might perform differently at 75 or 100 degrees (=burst temp?) than in my cold-water hydrotest tank. Thoughts? I’m going to have to make the fitting: is this a waste of time?
Also, I am looking forward to part III of the article! I do love technique and theory articles (and trip reports!) more than gear reviews…here’s hoping for more like this stove series.
Brian
Apr 10, 2006 at 6:51 am #1354537Brian,
Sorry for the delay in reply…I have been away on vacation :-)
If you have the time and capabilities, I would go ahead and do the test. You riase a good point about the temperature effects – but depending on what you see for burst temp, we can compensate somewhat for temperature, or otherwise use your results to help us judge whether we are to be most concerned about cannister failure, versus melting of the internal plastic components. You never really know what you will see, so it is usually a better path to go ahead and do an experiment…..
Oct 3, 2006 at 2:10 pm #1364173I recommend reading “The nature and behaviour of mixtures of fuels” by Roger Caffin for more reading on this topic. I found this enlightening when I first read it a while ago.
Oct 24, 2006 at 5:55 am #1365428Dondo,
You mentioned having a wind screen setup in the recent past. The picture is not showing up. Can you post it again?
Thanks,
Shane
Dec 22, 2006 at 9:01 am #1371967I just found this article and discussion in my search for winter camping cooking. Just curious, why don't they use higher mixes of propane in the gas canisters? Forgive me if this has been answered already and I missed it.
Dec 22, 2006 at 9:46 am #1371973I can give a partial answer–propane's vapor pressure is much higher than those of butane and isobutane. A pure propane canister is a heavy duty affair for this reason (e.g., the green steel Coleman propane canisters).
What I don't know is the maximum design pressure of standard steel Lindal cansiters, and what percentage of propane that pressure standard would represent.
Jan 4, 2007 at 10:30 pm #1373042Thanks for the comparisons and boil times. I'm in the market now for a winter stove and this has given me the direction I need.
Mar 12, 2007 at 1:16 am #1382005Why wouldn't we just use propane which has a low boiling point and would vaporize on it's own down to an extreme minus 40 degrees, why introduce Butane into the mix?
Mar 12, 2007 at 9:26 am #1382021>Why wouldn't we just use propane which has a low boiling point and would vaporize on it's own down to an extreme minus 40 degrees, why introduce Butane into the mix?
To quote Rick Dreher, above:
>> A pure propane canister is a heavy duty affair for this reason (e.g., the green steel Coleman propane canisters).A green steel Coleman propane canister holds 16.4 oz of fuel and weighs 31.4 oz–a canister weight of 15.0 oz. Two Coleman 70/30 butane/propane 220g canisters hold 15.5 oz of fuel and weigh 27.2 oz. Scaling the 70/30 canisters by the fuel weight ratio gives an equivalent canister weight of 12.4 oz per 16.4 oz of fuel, or about 2.5 oz more canister per pound of fuel for pure propane instead of 70/30. (I hate English units.)
I have a remote-canister liquid-fed stove (Coleman Exponent Xtreme) so I'm not too worried about my stove's cold-weather performance. But for winter camping, the extra weight of pure-propane canisters doesn't seem too much of a burden for the performance. Propane stoves, in addition to the big clunkers, are available in low-priced top-burner and remote-canister configurations, although I'm not sure how much they weigh. My personal experience with a dual-burner propane stove at merely freezing temps was not positive, so there may be other problems with using gas-feed propane (although I haven't had any problems with my gas grill at temps down to +10F).
Mar 14, 2007 at 11:19 am #1382267Another advantage of the 1-lb pure propane canisters is the availability of small catalytic heaters. If you're testing new winter gear (or a winter camper) you can pack a Coleman Survival Cat:
http://www.coleman.com/coleman/colemancom/detail.asp?product_id=5034A729&categoryid=3000
Coleman used to make a catalytic heater for the butane mix canisters, but for whatever reason it's off the market. I'd love to get my hands on one for trips with my girlfriend; it's a little bit of a buffer against error and fatigue.
Oct 28, 2010 at 9:14 am #1658894Roger – I think this article has missed a trick – with a remote canister stove you are no longer restricted to the squat canisters used for canister-top stoves. Any canister with a threaded Lindal valve can be used, eg


These are lighter (~85g empty) and cheaper than the squat 100g canisters but contain 170g or 175g gas (30% or 35% propane).
They are widely available in hardware stores (they are meant for small blow torches) and come in various brands – even CampingGaz now, their only threaded canister!Oct 28, 2010 at 1:56 pm #1659009Hi Stuart
I hadn't seen those!
BUT: do they have an internal tube to get a liquid feed when they are lying on their side? If not, they would be very hard to use. If yes … VERY interesting.
Cheers
Oct 29, 2010 at 1:25 am #1659209Roger – I'm pretty sure there is no internal tube.
They are very easy to use – raise the 'base' end by supporting it with something: boot/rock/snow/whatever so the canister is lying at an angle of 30* to 45*. There is no need to invert it vertically unless it is almost empty.Mar 15, 2011 at 3:00 pm #1709320I am wondering if the ETA packlite can be used with an inverted canister? Does the stove need to be up an running before inverting the canister?
Jul 27, 2011 at 3:50 pm #1763766The Eta Packlite has a pre heat loop, so you should be able to run it in inverted mode without a problem. I think Roger has reviewed it elsewhere on the site and that the review was favorable.
Generally, manufacturers recommend that the stove be started with the canister upright, the pre-heat tube allowed to warm, and then that the canister be inverted. In practice many of us have found that starting a stove with the canister inverted is no big deal if you go easy on the gas at first.
HJ
May 2, 2012 at 6:27 pm #1873748Coleman canisters seem to be hard come by. REI has discontinued them and when I called Coleman to question the availability they responded by saying they do not comment on pending litigation.
So what gives… If you have a current source please let me know. -
AuthorPosts
- You must be logged in to reply to this topic.
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Trail Days Online! 2025 is this week:
Thursday, February 27 through Saturday, March 1 - Registration is Free.
Our Community Posts are Moderated
Backpacking Light community posts are moderated and here to foster helpful and positive discussions about lightweight backpacking. Please be mindful of our values and boundaries and review our Community Guidelines prior to posting.
Get the Newsletter
Gear Research & Discovery Tools
- Browse our curated Gear Shop
- See the latest Gear Deals and Sales
- Our Recommendations
- Search for Gear on Sale with the Gear Finder
- Used Gear Swap
- Member Gear Reviews and BPL Gear Review Articles
- Browse by Gear Type or Brand.