Topic
Premixing AquaMira
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Home › Forums › General Forums › Philosophy & Technique › Premixing AquaMira
- This topic is empty.
-
AuthorPosts
-
Apr 2, 2013 at 7:14 pm #1301233
I first heard of premixing AquaMira from BPL, back when they sold dropper bottles from the BPL store. I was intrigued, but continued to mix one batch at a time. I carried extra mixing caps to mix multiple batches.
In 2010, I met a couple thru-hiking the PCT. They began their thru-hike using a filter, switched to AquaMira around Kennedy Meadows and soon tired of waiting five minutes before adding it to their water. They began premixing a whole set of AquaMira into the empty bottles from the previous set. As we hiked along together, they could scoop and treat water on the go while I was encumbered with the mixing and waiting process. Anecdotally, they hadn’t gotten sick and I began to consider premixing.
In 2011, Mike Clelland published his book “Ultralight Backpackin Tips” in which he describes premixing a day’s (or weekend; I can’t remember) supply of AquaMira. This additional encouragement led me to trying the practice.
I tried premixing a day’s supply but was still curious about premixing more which lead me to testing the parts per million concentrations of Chlorine Dioxide in water treated with premixed AquaMira.
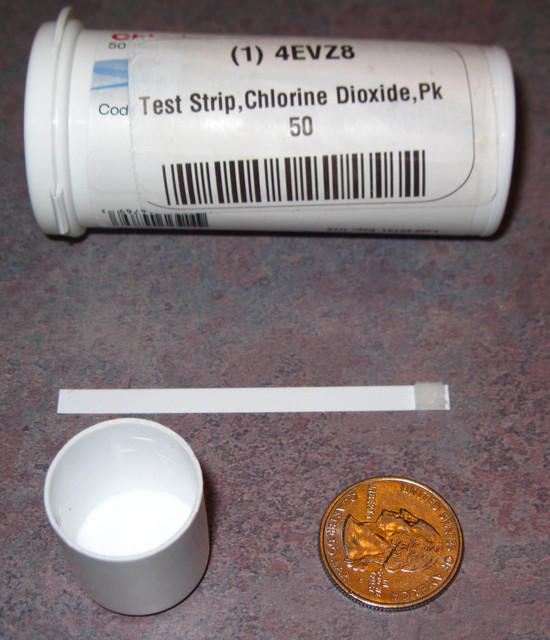
I purchased Chlorine Dioxide test strips formulated to measure 1 to 10 parts per million.
Next I premixed approximately 30 doses of AquaMira in an empty AquaMira bottle.
Over the next 33 days I compared the ppm concentration of Chlorine Dioxide in water treated with freshly mixed AquaMira against water treated with the premix.
Results:

I can only speculate why the CLO2 ppm in the premix was often greater than the CLO2 ppm in the fresh mix. Perhaps the fresh mix ‘off-gassed’ enough CLO2 during the 5 minute waiting period to account for the difference.Although not unexpected, I can only speculate why the premixed ppm dropped off. Was it because of the passage of time or did it ‘off-gas’ each time the bottle was opened? There are a lot of variables unaccounted for: temperature, humidity, wind, atmospheric pressure, exact drop size, etc.
USE THIS INFORMATION AT YOUR OWN RISK: IT IS ONLY ONE TEST OF CLO2 PPM IN WATER. IT IN NO WAY TESTS THE ACTUAL EFFECTIVENESS OF THE SOLUTION AGAINST PATHOGENS.

Typical results: Strip on right indicates greater CLO2 ppm than strip on left.

Hope this was interesting.
Apr 2, 2013 at 7:40 pm #1972182Nice write-up. Thanks for performing the experiment.
May 8, 2013 at 2:47 pm #1984521This is an elegant experiment. Well done. A corollary study could be done adding a known quantity of coliform bacteria (e.g. E. coli) to sterile water and seeing which method killed more bacteria. I may try this with my own preferred water treatment, a UV pen.
May 14, 2013 at 5:22 pm #1986119I have always limited myself to a single day of pre-mix. From what I understand the manufacturer wont even comment on anything but mixing for immediate use. This is really interesting. Thanks!
Sep 26, 2013 at 1:18 am #2028395Lance,
I've had the same question for a while when it comes to premixing Aquamira.It's nice that you did this test, but I suspect that your test strips might be too sensitive to actually tell you anything relevant, and this is probably why you are getting sort of weird results.
1 – 10 parts per million is an exceedingly small concentration of Chlorine Dioxide. I'm not sure what the exact concentration of chlorine dioxide is in the mixed (activated) Aquamira solution, but my guess is that it is at least several orders of magnitude higher than 10 ppm.
As such, you could have nearly all of the original chlorine dioxide break down through natural chemical decay over time and still get a positive result on your strip.
For example, if the initial activated solution is even 1% chlorine dioxide (1 part per hundred), then you would still get a positive reading on those strips even if there was only one ten-thousandth of the original chlorine dioxide present.
Is this making sense?
Part of the reason that I'm dubious of your conclusions is because it's already been pretty well established in the scientific literature that chlorine dioxide is a fairly short-lived chemical when exposed to the environment at room temperature.
One study pegged the half-life of chlorine dioxide in a municipal water supply at 93 minutes:
http://www.ncbi.nlm.nih.gov/pubmed/3028767?dopt=AbstractAssuming that this is relatively accurate, it would mean that after your activated Aquamira solution sits around for only 4 hours and 39 minutes, there will only be about 12.5% of the original chlorine dioxide left.
For all these reasons, I think that real test needed here is one that probes the exact concentration of chlorine dioxide in the activated solution over time. I'd really love to see that curve, but it would take a full chemistry lab to get this result.
I know McNett (maker of Aquamira) has this data, but I'm sure for legal reasons they aren't sharing it with consumers. Frustrating…
Sep 26, 2013 at 6:14 am #2028418I agree with Derek… The target concentration for Aqua Mira is roughly 5 ppm in the 1 liter of water after adding the mix to it. This means that the mix is closer to 5000 ppm, assuming you make about 1 mL to treat 1L (which may not be exact but is reasonably close.) That's way above the range of your test strips. You need to add the recommended dose to 1L of water and measure the ClO2 concentration in the water with your strips at each data point.
Sep 26, 2013 at 10:34 am #2028539Thanks Derek and Andrew.
"Over the next 33 days I compared the ppm concentration of Chlorine Dioxide 'in water treated with' freshly mixed AquaMira against 'water treated with' the premix."
I should have made this clearer by saying that each day I mixed the solutions with 1 liter of water per the Aqua Mira instructions to attain the target 5ppm.
The test strips with a range of 1 to 10ppm were just right!
In regards to one study pegging the half-life of chlorine dioxide in a municipal water supply at 93 minutes:
I don't doubt a short half life after diluting to 5ppm, however, I believe that the 10000 ppm premix concentration (.5ml per L water = 5ppm) in a closed container is much more stable and therefore has a much longer half life than after diluting to 5ppm in water.
I hope this helped.
Sep 26, 2013 at 10:42 am #2028543Ok Lance, that makes more sense, sorry for not reading more carefully. Do you have actual #s for what the measured premix concentration was each day instead of just greater than, less than, or equal?
A few of my coworkers have PhD's in physical chemistry. I have no knowledge of the chemistry of how ClO2 is produced from either the tabs or mix bottles, but if we have any intelligent questions (or maybe a paper I could reference?) I would be happy to talk to them. We also make a few gas analyzers that measure HCl concentrations but I am not sure if there's any way to use that for detection of ClO2.
Sep 26, 2013 at 10:53 am #2028548Sorry Andrew, I chose not to record actual ppm numbers. The 'color charts' on two different vials of test strips weren't quite the same. Also, after researching clo2 test strips, I concluded they weren't accurate enough for specific numbers, but fine for comparison between two tests using strips from the same batch. The cost of a more accurate clo2 test kit using liquid chemicals was too expensive for me.
More stuff:
I did play around with 2.5ppm and 10ppm and could see the difference on the test strips. The color on the strips after wetting faded noticeable over a few minutes, so I did the comparison strips simultaneously (within a few seconds of each other).-Lance
Sep 26, 2013 at 6:07 pm #2028690The chemistry isnt real straight forward. You can have multiple chlorine ionic species present. Without knowing what the test strips are responding to, its hard to say what is really being measured. I suppose I could figure it out, but Im too lazy.
However, I can say I think there is a big difference mixing a small amt of the AM in an open cup, and a large amount in a more closed container. I think quite a bit escapes during the 5 min wait time time in the open container when you follow directions.
This is part of the issue with certifying the drops as a purifier with the EPA, very hard to control the final resulting concentration.
I have mixed it by putting drops directly into the small black 3ml dropper, putting the lid on and shaking. If you do this, it comes out more concentrated it seems. The yellow tinge of the treated water is noticably darker, and the taste is stronger. I really think that mixing a bunch directly in the little black vial (with limited air exposure/volume ratio) results in a noticeablly stronger than intended premix. It has been hard to drink the water before due to the taste, especially if seal the top and shake to mix.
Sometimes when the premix is added after doing this way, the water will have a smoke haze above it when the drops hit it.
-
AuthorPosts
- You must be logged in to reply to this topic.
Forum Posting
A Membership is required to post in the forums. Login or become a member to post in the member forums!
Our Community Posts are Moderated
Backpacking Light community posts are moderated and here to foster helpful and positive discussions about lightweight backpacking. Please be mindful of our values and boundaries and review our Community Guidelines prior to posting.
Get the Newsletter
Gear Research & Discovery Tools
- Browse our curated Gear Shop
- See the latest Gear Deals and Sales
- Our Recommendations
- Search for Gear on Sale with the Gear Finder
- Used Gear Swap
- Member Gear Reviews and BPL Gear Review Articles
- Browse by Gear Type or Brand.